ABSTRACT
OBJECTIVES: to assess the association between pregnant women's consumption of ultra-processed foods and newborn body weight.
METHODS: prospective study with pregnant women (n=214) selected from all Basic Health Units in the city of Pinhais, Paraná. Socioeconomic, demographic, and health data were collected. Food consumption data were assessed using a 24-hour dietary recall and tabulated with GloboDiet software. Daily relative energy intake of ultra-processed food was estimated and logistic regression analysis was utilized. The influence of covariates on the association analysis was also explored (e.g., income and education).
RESULTS: ultra-processed foods contributed to 26.9% of pregnant women's total energy intake. About 5.7% of newborns were classified as small-for-gestational-age and 10.7% as large-for-gestational-age. A borderline statistically significant association was observed between large-for-gestational-age newborn weight and maternal consumption of ultra-processed foods (OR= 1.027; p=0.048). Additionally, family income was associated with the consumption of ultra-processed foods (OR= 0.144; p=0.008). With each additional 1% consumption of ultra-processed foods, mothers' likelihood of having large-for-gestational-age babies increased by about 2.7%.
CONCLUSION: the study reveals a trend of positive association between the weight of large-for-gestational-age newborns and the consumption of ultra-processed foods by pregnant women, but not for small-for-gestational-age children.
Keywords:
Eating, Processed food, Birth weight, Pregnant women, Health centers
RESUMO
OBJETIVOS: avaliar a associação entre o consumo de alimentos ultraprocessados por gestantes e o peso de recém-nascidos.
MÉTODOS: estudo prospectivo com gestantes (n=214) selecionadas em Unidades Básicas de Saúde em Pinhais, Paraná. Dados socioeconômicos, demográficos e de saúde foram coletados. Dados de consumo alimentar foram coletados por recordatório de 24-horas físico e entrados no software GloboDiet. O consumo diário relativo de energia proveniente de alimentos ultraprocessados foi estimado e a análise de regressão logística foi utilizada, considerando covariáveis como renda familiar e escolaridade.
RESULTADOS: o consumo de alimentos ultraprocessados pelas gestantes representou 26,9% da energia total. Cerca de 5,7% de recém-nascidos foram classificados como pequenos para idade gestacional (PIG) e 10,7% como grandes para idade gestacional (GIG). Foi observada uma associação estatisticamente significativa limítrofe entre o peso dos recém-nascidos GIG e o consumo materno de alimentos ultraprocessados (OR= 1,027; p=0,048). Além disso, a renda familiar esteve associada com o consumo de alimentos ultraprocessados (OR=0,144; p=0,008). A cada 1% adicional de consumo de alimentos ultraprocessados, a probabilidade de as mães terem recém-nascidos GIG aumentou cerca de 2,7%.
CONCLUSÃO: o estudo revela uma tendência de associação positiva entre o peso de recém-nascidos GIG e o consumo de alimentos ultraprocessados por mulheres grávidas, mas não para crianças PIG.
Palavras-chave:
Consumo de alimentos, Alimento processado, Peso ao nascer, Gestantes, Centros de saúde
IntroductionThe consumption of ultra-processed foods (UPF) has been discouraged for all populations due to their high content of energy, free sugar, salt/sodium, saturated fat, trans fats, and other substances that can be considered unhealthy.
1 In general, regular consumption of UPF in the diet can affect individuals' nutritional status and is associated with non-communicable chronic diseases (NCDs), such as cancer and related conditions.
2Therefore, it is important to limit the consumption of UPF during pregnancy and prioritize a healthy diet quality to enhance maternal and neonatal health.
3 During pregnancy, it is recommended to consume a variety of foods that provide adequate supplies of energy, proteins, vitamins, and minerals.
4 Insufficient or excessive dietary intakes can influence intrauterine fetal development and potentially result in inadequate birth weight among newborns.
5Birth weight, measured within the first hour of the newborn's birth, is an indicator of health conditions that reflect the nutritional status of both the pregnant woman and the newborn. It also has long-term implications for growth, development during childhood, and individual health conditions in adulthood.
6To date, few studies have examined food consumption during pregnancy based on the degree of food processing. Alves-Santos .
7 studied a cohort study on Brazilian women and found increased consumption of in natura/minimally processed foods during pregnancy, accompanied by reduced consumption of UPF. Another study involving mothers and newborns in the United States showed that the consumption of UPF was associated with increased gestational weight gain and a higher percentage of body fat in newborns.
3 Similarly, Gomes .
8 observed a positive association between UPF consumption in the third trimester of pregnancy among Brazilian women and the average weekly gestational weight gain.
However, we only found one study available that specifically focused on the association between UPF intake and newborn body weight.
9 In this study, a high consumption of in natura/minimally processed foods and culinary ingredients was considered a protective factor against the large-for-gestational-age (LGA) newborns. Conversely, medium to high consumption of UPF increased the chances of having a small-for-gestational-age (SGA) newborns.
Given the significance of body weight as a predictor of perinatal health, the influence of pre-pregnancy and during pregnancy food consumption on maternal and newborn health, and the limited information on this topic, the aim of this study was to assess the association between UPF consumption during the gestational period and newborn body weight.
MethodsThis was a prospective study conducted as part of the Multicenter Study of Iodine Deficiency (EMDI) in 11 cities across Brazil. The aim of the EMDI was to assess the nutritional status of Brazilian pregnant women, nursing mothers, and infants in relation to iodine, sodium, and potassium nutritional status.
10The specific study was carried out in the city of Pinhais, located in the metropolitan region of Curitiba, Paraná. Pinhais has an estimated population of 133,490 inhabitants as of 2020, with a Municipal Human Development Index (HDI) of 0.751 and an Index of Gini of 0.509.
11 Data collection took place in all 11 Basic Health Units (BHS) in Pinhais, ensuring representation from pregnant women who received direct assistance from the Unified Health System (SUS).
The study included pregnant women between the ages of 18 and 49 who were users of the BHS/SUS and provided informed consent. Pregnant women with thyroid disease (hypothyroidism, hyperthyroidism, Hashimoto's disease, and neoplasms) or those who had undergone thyroid gland surgery were excluded. These were criteria of the main multicenter study.
Initially, 305 pregnant women were approached, and 282 were found to be eligible. Out of these, 272 had complete and plausible sociodemographic, health, and food consumption data. Finally, a total of 214 pregnant women were included in the analysis as they had newborn data, including body weight, gender, and maternal gestational age at birth.
While an initial sampling strategy was defined for the multicenter study,
10 the sample size for this specific research was subsequently calculated. The objective was to test whether the odds ratio between body weight adjusted for gestational age and the consumption of UPF foods was different from 1. The PSS Health tool online version
12 was used for this calculation, considering a significance level of 5%, power of 80%, and an expected odds ratio of 1.5. The estimated sample size of 191 subjects was deemed sufficient.
Data collection occurred from March 2019 to March 2020. Pregnant women were approached in the waiting room of the BHS while waiting for assistance. In a few cases, interviews were conducted during home visits. The data collection team received two days of training from the national coordination of the study, which included specific training on the 24-hour dietary recall (R24h).
Socioeconomic, demographic, and lifestyle data were collected through a structured questionnaire incorporated into the REDCap application. Afterwards, data on food consumption were collected using the R24h method, with the aid of the Multiple Pass Method (MPM). A paper-based version of the recall was employed.
13 This R24h was modified to allow for a more comprehensive classification of foods based on their degree of processing (i.e. including details such as type of food processing: homemade or industrialized). Furthermore, it included a dedicated space where participants could provide detailed information about recipes, including the ingredients used and their respective amounts, if known. Additionally, the "Brazilian Manual for Food Portion Quantification"
14 was utilized as a resource for portion quantification.
One R24h was administered to the entire sample, and a second recall was conducted in a subsample (18%) with a minimum interval of one week. These recalls were obtained on various days of the week, with 81.7% of them representing food consumption from monday to thursday. Furthermore, they were collected during different seasons, with 24.3% in spring, 25.3% in summer, 28.0% in autumn, and 22.4% in winter.
Other data collected included birth weight, the sex of the baby, and gestational age on the delivery day. This information was acquired from the Health Department of the city of Pinhais. In instances where such data was unavailable (n=56), telephone contact was established with the mothers to gather the information after the birth of the babies.
Food consumption data were inputted into the GloboDiet software, Data Entry version, which had been adapted for the Latin American context.
15 This software was developed as part of a global initiative to adapt a computerized version of the R24h. The software generated notes highlighting inconsistencies or missing information. Consequently, after entering the R24h data, a data quality control process was conducted by addressing the generated notes. Inconsistencies in the description or quantification of food consumption were treated in a standardized way as per the guidelines in the "Standardization Manual of the Treatment of Notes in GloboDiet" developed by the research group.
13The foods consumed were classified according to the NOVA classification: in natura/minimally processed, processed culinary ingredients, processed foods, and ultra-processed foods.
16 Additionally, the classification of foods reported was carried out following the specifications of the base document developed by the Dietary Exposure Research Group of the Department of Nutrition at UFPR.
13 Thus, the classification according to NOVA was performed with the addition of a fifth category called "uncertainty" to avoid allocating a food item to a specific group due to lack of detailed information provided in the dietary recall. For the final classification, foods that were still classified as uncertain due to a lack of clarity regarding consumption patterns in the center (e.g., unspecified cake) were categorized with the lowest level of processing possible, considering the least conservative scenario, as proposed by EFSA, to address uncertainty scenarios in dietary exposure assessment.
17The classification of foods allowed for the quantification of energy intake from UPF and estimation of its proportion in relation to total energy consumption. The Brazilian Food Composition Table
18 was employed to estimate the energy composition of foods. Both the data linkage to food composition and the classification of foods according to NOVA were conducted by two researchers at the level of food disaggregation and recipe ingredient analysis. At the end, the daily energy contribution of each pregnant woman was calculated. In cases where pregnant women had two recalls, the average of the two days was used. Additionally, the proportion of means method was applied to evaluate the contribution of foods to the energy intake derived from ultra-processed foods, identifying the top ten most consumed items.
To evaluate the plausibility of R24h, dietary reports below 500 Kcal/day, above 4000 Kcal/day,
19 or with fewer than five food items were reviewed. A biological plausibility criterion was applied to determine their inclusion, accepting reports of nausea, vomiting, excessive appetite, or increased consumption due to an atypical day. In total, 4 R24h were considered implausible.
The classification of newborn weight adequacy, based on gestational age and sex, was conducted using the Intergrowth-21
st curves.
20 Newborns were categorized as SGA if their weight fell below the 10
th percentile, as adequate for gestational age (AGA) if their weight fell between the 10
th and 90
th percentiles, and as LGA if their weight exceeded the 90
th percentile.
20,21Mean and standard deviations or confidence intervals were calculated for continuous variables, while absolute and relative frequencies were estimated for categorical variables. The effect of UPF consumption on the birth weight to the gestational age was evaluated through logistic regression analysis. Besides the UPF consumption, the following covariates were also considered: age (in years), race (white/yellow or brown/black), family income Brazilian real (BRL) - (<BRL 1,000.00, BRL 1,000.00 to BRL 3,000.00, and ≥BRL 3,000.00), maternal educational level (incomplete/completed elementary school, incomplete/completed high school, incomplete/completed higher education), smoking status (yes or no), alcohol intake habit during pregnancy (yes or no), cohabitation with a spouse (yes or no), parity (=1 or >1 child), pre-pregnancy Body Mass Index (BMI) (non-excessive body weight (< 25 kg/m
2) and excessive body weight (≥ 25 kg/m
2), gestational trimester (first, second, and third). The BMI values were categorized according to the criteria of the Center for Disease Control and Prevention,
22 including pregnant adolescents (≤ 19 years old) in the sample (n=11). Overweight and obesity cases were combined into the category of excessive body weight. This approach was taken as the results remained consistent when compared to a more specific classification for this age group.
The odds of small and large weight for gestational age were estimated. The results are presented as estimated odds ratios, relative to adequate weight for gestational age, along with their corresponding 95% confidence intervals. Robust standard errors were calculated to account for possible model misspecifications. Statistical significance was assessed using the Wald test, and sample weights were included to ensure accurate estimation of the parameters of interest.
Unadjusted odds ratios were calculated for both small and large weight for gestational age, while adjusted odds ratios were obtained only for large weight for gestational age, as the covariates did not show a significant effect when adjusted for the others. The adjusted odds ratios were derived using multiple logistic regression. The covariates included in the analysis were determined using the following procedure: first, the covariates with <0.20 in the unadjusted analysis were considered for the multiple regression model. Then, a backward selection procedure was conducted to eliminate the covariates with <0.10 in the adjusted analysis.
All analyzes were carried out using the R software for statistical computing, version 4.0.2. The R library "survey" was used for model fitting, aiming to introduce the sample weights and obtain the robust estimates.
23The EMDI-Brazil study received approval from the Research Ethics Committees of the Federal University of Viçosa (coordinator; nº 2,496,986) and the Ethics Committee of the Federal University of Paraná (co-participant; nº 2.802.098).
ResultsThe maternal and neonatal characteristics, based on the adequacy of birth weight for gestational age, are presented for the pregnant women and their newborns in Table 1. The mean maternal age was 26.4 years, the pre-gestational BMI averaged 28.2 kg/m², and the gestational age at birth averaged 38.6 weeks. When compared to the mothers of newborns with AGA, the mothers of SGA and LGA newborns showed slightly higher maternal age and smaller pre-gestational BMI and gestational age at birth. Furthermore, 52.7% of the women were classified as having excessive body weight, 50.4% were brown or black, 61.6% attended high school, 83.1% lived with a spouse, 56.9% earned between 1000 and 3000 BRL, 39.3% were in the first trimester of pregnancy, 4.9% smoked, 1.5% had the habit of alcohol intake, and 62.6% had given birth to more than one child. The proportion of mothers with higher and lower education levels was slightly different among the groups of SGA, LGA, and AGA children. Similarly, a higher proportion of mothers of LGA children had either a smaller or higher monthly income compared to those of SGA and AGA.
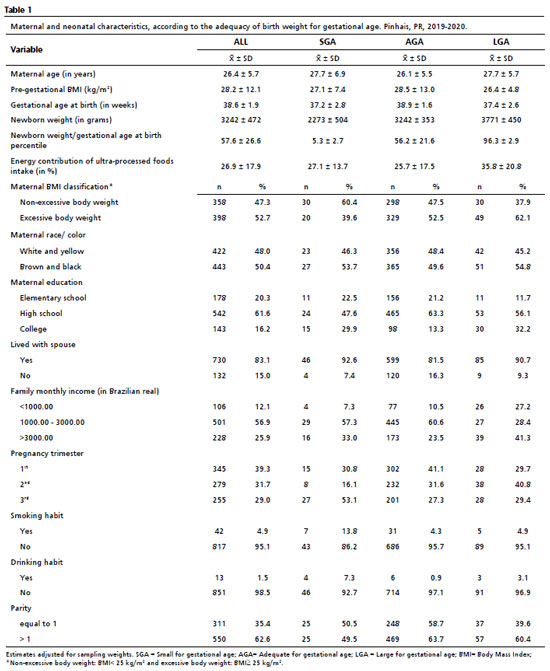
The mean birth weight was 3,240 kg (3,206-3,274), with SGA newborns having a mean weight of 2,273 kg (2,130-2,417), and LGA newborns having a mean weight of 3,721 kg (3,621-3,823).
The consumption of UPFs by the women accounted for 26.9% (25.7-28.0) of the total energy intake. Pregnant women who gave birth to LGA newborns had a higher UPF consumption (35.8%; 31.6-40.1) compared to SGA (27.1%, 23.2-31.0) and AGA (25.7%, 24.4-27.0). The ten most frequently consumed UPFs included cola-type soda, cheese bread, margarine, biscuits, unspecified soda, non-specified sausage, non-specified cracker biscuits, industrialized fruit juice, hot dogs and industrialized bread (data not tabulated). Together, these food items contributed to 36.1% of the reported energy intake derived from ultra-processed foods.
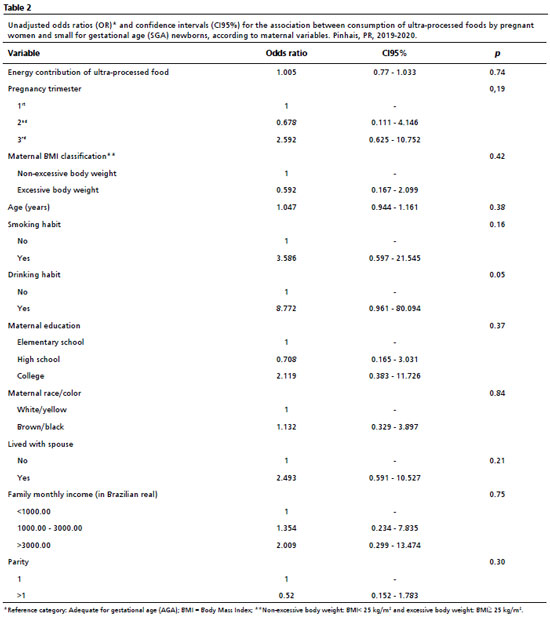
When analyzing the birthweight adjusted for gestational age and the consumption of UPF, no significant association was found with SGA newborns (Table 2). None of the considered covariates showed a significant association with a small weight for gestational age. However, in the unadjusted analyses of LGA newborns (Table 3), we observed a potentially significant association, on the border of statistical significance, between birth weight adjusted for gestational age and the consumption of UPF (OR= 1.028; CI95%= 1.000-1.058; =0.05), level of education (OR= 4.394; CI95%= 1.147-16.835; =0.07), and family income (OR= 0.181; CI95%= 0.050-0.659; =0.013). This possibly indicated that the chance of giving birth to an LGA newborn increased by 2.8% with each additional 1% of UPF consumed by the pregnant woman. Additionally, the chance of giving birth to an LGA newborn was estimated to be 4.44 times higher for mothers with incomplete/complete higher education compared to those with incomplete/complete primary education, and 0.181 times for those with an income range between 1,000 to 3,000 compared to those with an income of up to 1,000 BRL.
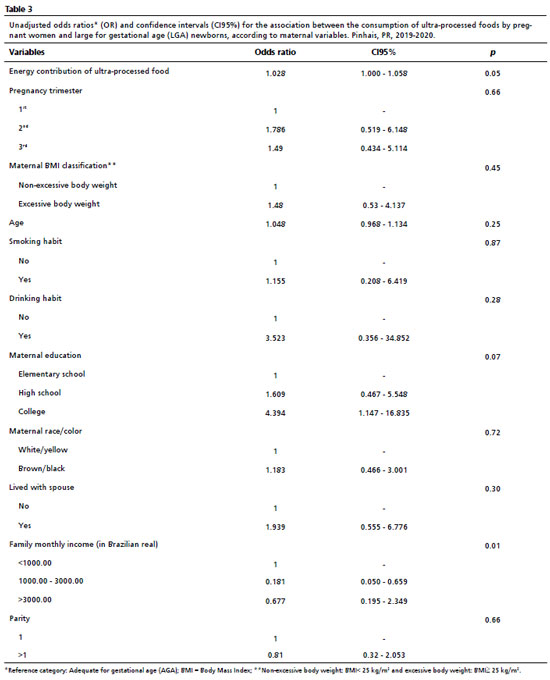
When the data were analyzed for the group of LGA children in adjusted effects analyzes (Table 4), a trend association was found between the consumption of UPF by pregnant women and the birth weight of the newborns (OR= 1.027; CI95%= 1.000-1.054; =0.048). Additionally, family income (OR= 0.144; CI95%= 0.042-0.492; =0.008) was shown to be associated with UFP consumption. The chance of mothers having LGA babies increased by approximately 2.7% for each additional 1% consumption of UPF. Furthermore, the chance of an LGA newborn was 0.14 times lower for those who had an income range between 1,000 and 3,000 compared to those with an income range up to 1,000 BRL. On the other hand, the chance of giving birth to an LGA newborn was estimated to be 6.1 times higher for mothers with incomplete/completed higher education compared to those with incomplete/completed elementary education although this was not of statistical significance.
DiscussionThis study examined the relationship between UPF consumption in pregnant women and birth weight of newborns adjusted for gestational age. The findings revealed a trend of positive association between UPF consumption by pregnant women and the birth weight of LGA newborns, as well as family income. In contrast to the only study examining the association between UPF consumption and newborn birth weight,
9 the present study did not find evidence that moderate to high UPF consumption during pregnancy increased the likelihood of SGA newborns.
A longitudinal study conducted in Rio de Janeiro examined the dietary habits of pregnant women and categorized their food consumption into four patterns.
24 Although different from our research, one of the patterns identified was labeled as the Western pattern, accounting for 6.9% of the total consumption (including items such as potato/cassava/yam, pasta, flour/farofa/polenta, pizza/burger/pastel, soda/soft drink, and pork/hotdog/sausage/egg). Another pattern was referred to as the snack pattern, which explained 5.7% of the variation in consumption (consisting of stuffed biscuits, savory biscuits, and chocolate). Interestingly, the study found that greater adherence to the snack food pattern among teenage mothers during pregnancy was associated with higher birth weight of their newborns.
Miranda .
25 conducted a systematic review of the influence of dietary exposures, somewhat related to the consumption of UPFs during pregnancy, on the anthropometric parameters of children up to one year of age. The review examined outcomes such as birth weight and its classifications, and adequacy of birth weight according to gestational age, among others. Overall, the review found non-significant associations between the dietary exposures (mostly "ultra-processed" food pattern; soft drinks, artificially sweetened beverages, and beverages sweetened with sugar; "fast food," "junk food," sweets, and snacks) and the anthropometric measurements, including birth weight (n=9), SGA (n=5), and LGA (n=4). It also identified a limited number of both direct and inverse associations between the exposures and the outcomes. According to the authors, the observed divergences in results could be attributed to methodological diversity among the studies, encompassing variations in sample populations, types and timing of dietary assessments, and the presence of comorbidities. However, it is noteworthy that this review highlighted the findings of two studies indicating that an inadequate dietary pattern (specifically based on fast food and sweets) could elevate the likelihood of LGA, aligning with observations in our study.
The proportion of UPF consumption among pregnant women in this study was lower (26.9%) compared to other studies conducted with Brazilian pregnant women, which reported values of 32% and 41.3%.
7,26 It is worth noting that Mariano .
27 conducted a study using data from the Brazilian National Food Consumption Survey (POF 2017/2018) and found a lower UPF consumption of 20.9% among pregnant women. However, the POF data does not specifically statistically represent the pregnant Brazilian population. In addition, when examining the other centers participating in the EMDI study, of which our study is a part, Pinhais was the center with the highest energy contribution from UPF consumption as compared to the other ten study centers.
28Furthermore, the consumption of UPF in the general Brazilian population assessed at POF was estimated at 19.7% of total calories. The ten most consumed UPFs were margarine (2.8%), salty biscuits and packaged snacks (2.5%), bread (2.1%), sweet biscuits (1.7%), cold cuts and sausages (1.6%), ice cream/jelly/flan/industrialized dessert (1.4%), soft drinks (1.3%), hot dogs/hamburgers/other sandwiches (1.1%), dairy drinks (1.1%), and pizza (0.9%),
29 contributing to a total of 16.5%. In contrast, it's noteworthy that ten specific foods accounted for approximately 36% of the energy contribution from UPF in the present study, with cola-type soda (5.1%) emerging as the primary contributor to energy consumption within the ultra-processed food category.
With that said, it is crucial to recognize that the consumption of UPFs leads to an overall elevation in calorie intake. Consequently, this intake can lead to unfavorable pregnancy and newborn health outcomes, such as excessive adiposity, type II diabetes, cardiovascular diseases, mental health issues, and cancer. Rohatgi .
3 revealed that higher energy consumption from ultra-processed foods was significantly associated with increased weekly gestational weight gain and neonatal anthropometrics (subscapular and thigh skinfold thicknesses, as well as the body fat percentage), independent of the mother's pre-pregnancy nutritional status. For newborns, increased body fat could be a predictor of obesity in adulthood. Therefore, the consumption of ultra-processed foods should be limited during pregnancy, and the focus of prenatal care should be on improving maternal and newborn health by emphasizing a diet rich in fresh or minimally processed foods and promoting of homemade meals.
In this study, it was observed that pregnant women with family income ranging from 1000 to 3000 BRL had a decreased likelihood of consuming UPF among LGA newborns. The association between income and higher consumption of UPF has been supported by previous research. It is recognized that the dietary pattern centered around UPFs remains more expensive compared to the pattern emphasizing fresh or minimally processed foods in Brazil.
28 However, our study did not find any association between UPF consumption and income exceeding 3000 BRL when assessing children with SGA and LGA. Furthermore, it is unclear why this association was only observed for LGA children, and not SGA groups. This lack of association remains to be explored.
The present study has some limitations. Firstly, the information collected on food consumption using the R24h method relies on the memory and cooperation of the respondents, which can lead to underestimation of the data quality. Second, the lack of correction for intra-individual variability may have influenced the detection of associations. While we collected two R24hs from a subset of pregnant women (18% of the sample), we did not perform the correction for intrapersonal variability as it would not allow for the calculation of the energy contribution percentage used in this study. Last, this research was a cross-sectional study, where exposure and outcome data are collected at the same time, preventing the establishment of a causal relationship between them.
Nevertheless, this research also possesses strengths. The study is part of a multicenter investigation involving a representative population of Pinhais in the Parana state. The study population consisted of pregnant women attending the basic health units of the Brazilian Unified Health System. Furthermore, a notable aspect of this research is the high quality of data collected using the R24h method, employing a multiple step approach and quantifying portions with the assistance of a food quantification album, subsequently systematized using the GloboDiet software.
As a conclusion, the results of this study demonstrate a trend of a positive association between the weight of LGA newborns and the UFP consumption during pregnancy, with higher UPF consumption being associated with a family income ranging from 1,000 to 3,000 BRL.
References1. Louzada MLdC, Martins APB, Canella DS, Baraldi LG, Levy RB, Claro RM,
et al. Ultra-processed foods and the nutritional dietary profile in Brazil. Rev Saúde Pública. 2015; 49: 1-11.
2. Isaksen IM, Dankel SN. Ultra-processed food consumption and cancer risk: A systematic review and meta-analysis. Clin Nutr. 2023; 42: 919-28.
3. Rohatgi KW, Tinius RA, Cade WT, Steele EM, Cahill AG, Parra DC. Relationships between consumption of ultra-processed foods, gestational weight gain and neonatal outcomes in a sample of US pregnant women. Peer J. 2017; 5: e4091.
4. WHO. WHO recommendations on antenatal care for a positive pregnancy experience: Geneva: WHO; 2016. [access in 2022 Mar 2]. Available from:
https://www.who.int/publications/i/item/97892415499125. Padilha PdC, Saunders C, Machado RCM, Silva CLd, Bull A, Sally EdOF,
et al. Associação entre o estado nutricional pré-gestacional e a predição do risco de intercorrências gestacionais. Rev Bras Ginecol Obstet. 2007; 29: 511-8.
6. Schellong K, Schulz S, Harder T, Plagemann A. Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PloS one. 2012; 7: e47776.
7. Alves-Santos NH, Eshriqui I, Franco-Sena AB, Cocate PG, Freitas-Vilela AA, Benaim C,
et al. Dietary intake variations from pre-conception to gestational period according to the degree of industrial processing: A Brazilian cohort. Appetite. 2016; 105: 164-71.
8. Gomes CB, Malta MB, Benício MHD, Carvalhaes M. Consumption of ultra-processed foods in the third gestational trimester and increased weight gain: a Brazilian cohort study. Public Health Nutr. 2021; 24: 3304-12.
9. Rocha GG, Andrade-Silva A, Alves-Santos NH, Castro MBTd. Association between maternal dietary intake classified according to its degree of processing and sex-specific birth weight for gestational age. Rev Nutr. 2022; 35: e210197.
10. Silva DLF, Crispim SP, Almeida CCB, Schrubbe V, Azevedo FM, Faria FR,
et al. Improving Pregnant Women´s Iodine Intake Estimates and Its Prevalence of Inadequacy through the Use of Salt and Seasoning Covariates. Nutrients. 2023; 15: 1-16.
11. Instituto Brasileiro de Geografia e Estatística (IBGE). Censo demográfico 2010-2011. [access in 2022 Mar 2]. Available from:
https://censo2010.ibge.gov.br/resultados.html12. Borges RB, Mancuso ACB, Camey SA, Leotti VB, Hirakata VN, Azambuja GS,
et al. Power and Sample Size for Health Researchers: uma ferramenta para cálculo de tamanho amostral e poder do teste voltado a pesquisadores da área da saúde. Clin Biomed Res. 2020; 40: 247-53.
13. Crispim SP, Silva DLF, Macedo MdS, Almeida CCB, Elias VCM, Franceschini SdCC. Aspectos metodológicos na avaliação do consumo alimentar de gestantes no Estudo Multicêntrico de Deficiência de Iodo. Rev Nutr. 2024 (
in press).
14. Crispim SP, Fisberg RM, Almeida CCB, Nicolas G, Knaze V, Pereira RA,
et al. Manual Fotográfico de Quantificação Alimentar. Curitiba: UFPR; 2017. 147p.
15. Bel-Serrat S, Knaze V, Nicolas G, Marchioni DM, Steluti J, Mendes A,
et al. Adapting the standardised computer- and interview-based 24 h dietary recall method (GloboDiet) for dietary monitoring in Latin America. Public Health Nutr. 2017; 20: 2847-58.
16. Monteiro CA, Cannon G, Levy RB, Moubarac JC, Louzada ML, Rauber F,
et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019; 22: 936-41.
17. European Food Safety Authority (EFSA) Scientific Committee, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S,
et al. Guidance on uncertainty analysis in scientific assessments. EFSA J. 2018; 16 (1): e05123.
18. Tabela Brasileira de Composição de Alimentos (TBCA). Universidade de São Paulo (USP). Food Research Center (FoRC). Versão 7.2. São Paulo; 2023. [access in 2021 Aug 01]. Available from:
http://www.fcf.usp.br/tbca19. Willett W. Nutritional epidemiology. 3
rd ed. New York: Oxford University Press; 2012.
20. Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG,
et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014; 384: 857-68.
21. World Health Organization (WHO). Guidelines on optimal feeding of low birth-weight infants in low-and middle-income countries. Geneva: WHO; 2011. [access in 2022 Mar 2]. Available from:
https://www.who.int/publications/i/item/978924154836622. Centers for Disease Control and Prevention (CDC). Defining Adult Overweight and Obesity. USA: CDC; 2017. [access in 2022 Mar 1]. Available from:
https://www.cdc.gov/obesity/basics/adult-defining.html23. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria; 2020. [access in 2022 Mar 1]. Available from:
https://www.r-project.org/24. Coelho NdLP, Cunha DB, Esteves APP, Lacerda EMdA, Theme MM. Dietary patterns in pregnancy and birth weight. Rev Saúde Pública. 2015; 49: 1-9.
25. Miranda C, Souza RCV, Santos LCd. Influência do consumo de alimentos ultraprocessados durante a gestação nas medidas antropométricas do bebê, do nascimento ao primeiro ano de vida: uma revisão sistemática. Rev Bras Saúde Matern Infant. 2021; 21: 9-26.
26. Sartorelli DS, Crivellenti LC, Zuccolotto DCC, Franco LJ. Relationship between minimally and ultra-processed food intake during pregnancy with obesity and gestational diabetes mellitus. Cad Saúde Pública. 2019; 35: e00049318.
27. Mariano KdR, Andrade GC, Louzada MLC, Nakamura MU, Araujo Júnior E, Souza E. Ultra-processed foods and the nutritional quality of the diet of Brazilian pregnant women. Rev Assoc Med Bras. 2023; 69: 169-74.
28. Silva GB. EMDI-BRASIL: O quê, quando, quanto e onde comem as gestantes brasileiras atendidas na atenção básica à saúde? [Dissertação]. Curitiba: Programa de Pós-graduação Alimentação e Nutrição da Universidade Federal do Paraná; 2021.
29. Instituto Brasileiro de Geografia e Estatística (IBGE). Pesquisa de Orçamento Familiar. Pesquisa Nacional de Consumo Alimentar 2017/2018. Brasília (DF): IBGE; 2019. [access in 2022 Mar 2]. Available from:
https://www.ibge.gov.br/estatisticas/sociais/saude/24786-pesquisa-de-orcamentos-familiares-2.htmlAuthor's contribution: Crispim SP, Almeida CCB, Macedo MS, and Franceschini SCC were responsible for the design and funding of the study. Schrubbe V, Silva DLF, Elias VCM, and Taconeli CA were involved in tabulating and statistically analyzing the information. Crispim SP and Schrubbe V drafted the first version of the manuscript.
All authors approved the final version of the article and declare no conflict interest.
Received on June 14, 2023
Final version presented on April 3, 2024
Approved on April 11, 2024
Associated Editor: Sheila Costa
 ; Débora Letícia Frizzi Silva2
; Débora Letícia Frizzi Silva2 ; Claudia Choma Bettega Almeida3
; Claudia Choma Bettega Almeida3 ; Cesar Augusto Taconeli4
; Cesar Augusto Taconeli4 ; Vanessa Cardozo Mendes Elias5
; Vanessa Cardozo Mendes Elias5 ; Mariana de Souza Macedo6
; Mariana de Souza Macedo6 ; Sylvia do Carmo Castro Franceschini7
; Sylvia do Carmo Castro Franceschini7 ; Sandra Patricia Crispim8
; Sandra Patricia Crispim8