ABSTRACT
OBJECTIVES: to investigate the relation between insulin use, pregestational nutritional status, and maternal weight gain during pregnancy among women with gestational diabetes mellitus.
METHODS: a retrospective cross-sectional study (2017-2022) was conducted with data from women followed at a University hospital in the Southeast of Brazil. Maternal weight gain adequacy was assessed according to new curves proposed for Brazilian pregnant women. Pregestational nutritional status, age, height, race, schooling, marital status, residence, parity, number of pregnancies and abortions, fasting glucose, and hypertensive disorders were analyzed. The use of insulin was considered the outcome in a multiple logistic regression model, using as reference, the categories least associated with gestational diabetes mellitus.
RESULTS: among the 353 participants, 48.7% were obese and 47.4% required insulin. Obese women were more likely to use insulin (OR= 2.26; 95%CI= 1.16-4.41; p=0.003). Regarding to weight gain, eutrophic women with above-adequate gain had greater odds of insulin use (OR= 3.22; 95%CI= 1.15-9.05; p=0.024). Conversely, among those with adequate or below-adequate gain, obese women showed higher chances of needing insulin (OR= 4.33; 95%CI= 1.76-10.6; p=0.001).
CONCLUSION: the findings reinforce the necessity for specific guidelines on gestational weight gain for women with gestational diabetes mellitus, considering differences in both nutritional status and weight gain patterns throughout pregnancy.
Keywords:
Maternal weight gain, Gestational diabetes mellitus, Insulin therapy
RESUMO
OBJETIVOS: investigar a relação entre uso de insulina, estado nutricional pré-gestacional e ganho de peso materno durante a gestação de mulheres com diabetes mellitus gestacional.
MÉTODOS: estudo transversal retrospectivo (2017–2022) com dados de mulheres atendidas em um hospital universitário do sudeste do Brasil. A adequação do ganho de peso foi avaliada segundo curvas específicas para gestantes brasileiras. Foram analisados estado nutricional pré-gestacional, idade, estatura, raça, escolaridade, situação conjugal, localidade, paridade, número de gestações e abortos, glicemia de jejum e distúrbios hipertensivos. O uso de insulina foi considerado desfecho em modelo de regressão logística múltipla, adotando-se como referência as categorias menos associadas ao diabetes mellitus gestacional.
RESULTADOS: entre 353 participantes, 48,7% eram obesas e 47,4% utilizaram insulina. Obesas apresentaram maiores chances de uso de insulina (OR= 2,26; IC95%= 1,16–4,41; p=0,003). Quanto ao ganho de peso, eutróficas com ganho acima do adequado tiveram maior chance de utilizar insulina (OR= 3,22; IC95%= 1,15–9,05; p=0,024). Entre as que tiveram ganho adequado ou abaixo do adequado, obesas mostraram maiores chances de necessitar insulina (OR= 4,33; IC95%= 1,76–10,6; p=0,001).
CONCLUSÃO: os achados reforçam a necessidade de diretrizes específicas de ganho de peso para mulheres com diabetes mellitus gestacional, considerando diferenças de estado nutricional e padrão de ganho durante a gestação.
Palavras-chave:
Ganho de peso materno, Diabetes melittus gestacional, Insulinoterapia
IntroductionWorld widely, Gestational Diabetes Mellitus (GDM) accounts for approximately 75–90% of diabetes cases during pregnancy,
1 contributing to the growing epidemic of type 2 diabetes mellitus (T2DM). About half of the women with GDM in the world may develop T2DM within 10 years after pregnancy and/or give birth to children with an increased risk of developing diabetes or obesity in the future.
2 Between 2014 and 2019, GDM accounted for 15.32% of maternal deaths in Brazil, ranking as the third leading cause.
3 Although data on the prevalence of GDM in Brazil remain limited and sometimes conflicting, a meta-analysis using current diagnostic criteria estimated an 18% prevalence among adult women,
4 with approximately 10% of these cases attributed to obesity.
5On the other hand, obesity affects one-third of the women of reproductive age,
6 with its prevalence doubling among 20-year-old women between 2003 and 2019, rising from 14.5% to 30.2%.
7 Women with obesity face an increased risk of mortality during pregnancy,
8 and Body Mass Index (BMI) is the main parameter associated with GDM in several studies identifying a connection between previous overweight and obesity, or inadequate gestational weight gain with its development.
9 Even so, the most commonly used weight gain recommendations are even proposed for pregnant women with overweight or obese ,
10 inclusively in recent studies, all of which were conducted by healthy women without diseases or disorders that could compromise maternal weight.
11,12 Pregnant women with diabetes follow the same weight gain guidelines as others,
13 with few studies assessing specific consequences and alternative treatments. Additionally, these women must monitor their blood glucose levels throughout their pregnancy, following established targets to improve the outcomes.
14The first treatment for pregnant women with GDM is nutritional therapy combined with encouragement of physical exercise for weight control and, consequently, improved glycemic control. Pharmacological insulin therapy is initiated only, when necessary, for those who do not achieve glycemic targets by dieting and exercising alone.
15 Therefore, it is implied that pregnant women who do not control their weight gain during pregnancy and/or were getting pregnant by being overweight or obese are more likely to experience poor glycemic control, thus having insulin therapy more frequently is required when compared to others. In this sense, the present study aimed to investigate the relation among insulin use (IU), pre-pregnancy nutritional status (PNS) and maternal weight gain (WG) during pregnancy among women with gestational diabetes mellitus (GDM).
MethodsThis is a retrospective cross-sectional study using secondary data collected from pregnant women's with GDM electronic medical records treated from 2017 to 2022 at a high-risk prenatal care service in a University hospital in the Southeast of Brazil. A total of 464 medical records were analyzed, of which 355 women aged 18 or older with GDM in singleton pregnancies and complete relevant data in their records were included. Exclusion criteria were those under 18 years of age, had other types of diabetes, multiple pregnancies, and the lack of glycemic control during prenatal care. After applying the exclusion criteria, 109 women were excluded from the study, as described in Figure 1.
The variables studied included the following sociodemographic characteristics: age (years), marital status (single, widowed, divorced, married, or stable union), schooling level (less than high school, completed high school, higher education), skin color (Black, Mixed, white),
9 and residence location (countryside of the State, metropolitan region, capital city).
Among anthropometric characteristics, height (up to 160cm, above 160cm),
9 pre-pregnancy nutritional status (PNS) (underweight, eutrophic, overweight, and obesity), and gestational weight gain (GWG) were collected, classified according to the criteria of the new curves for Brazilian pregnant women
12 and divided into two categories: those within or below the adequacy percentiles and those above the adequacy percentiles.
Clinical and obstetric characteristics included gestational age, parity (nulliparous or with at least one previous pregnancy), number of pregnancies (primigravida or with two or more pregnancies), miscarriages (yes/no), fasting blood glucose (above or below 92mg/dl), presence or history of hypertensive disorders (yes/no), and, as the outcome variable of interest, the use of insulin as an adjunct therapy for GDM treatment.
The collected data were entered and stored in a database created using Microsoft Office Excel 2016. For statistical analyses, the data were transferred to the Statistical Package for Social Sciences (SPSS) for Windows, version 22.0. The sample was characterized by means of frequency distribution and estimation of measures of central tendency and dispersion.
Associations between categorical variables were assessed using Pearson's chi-square test and/or Fisher's exact test, adopting a 5% significance level. Multivariate logistic regression models were tested to evaluate the effect of independent variables on insulin use (IU). Crude and adjusted odds ratios (OR) were calculated, with 95% confidence intervals.
In the multivariate regression models, the reference category for each independent variable tested was the one theoretically least associated with IU
9. For the construction of the multiple linear model, variables with
p≤0.20 in the bivariate analysis were included. In the final model, the backward method was used, in which variables with lower significance (higher
p-value) were removed one by one. This procedure was repeated until all the present variables in the model showed statistical significance (
p<0.05).
This present study followed the guidelines of Resolution CNS 466/12 of the Brazilian Ministry of Health (BRASIL, 2012) and was approved by the Research Ethics Committee of the
Universidade Federal do Espírito Santo (Federal University of Espírito Santo) under protocol N
o. CAAE: 66626222.0.0000.5060 on December 13, 2022, as well as by the Research Network of the EBSERH system under protocol No. 23525.017280/2022-95, SEI No. 25914497, on November 28, 2022.
ResultsTable 1 presents the characteristics of the sample of 355 pregnant women with GDM, with a mean age of 31±6.4 years, mean pre-pregnancy weight of 79.8±18.7 kg, and a mean WG of 7.91 ±7.06 kg by the end of pregnancy at 36±4 weeks of gestation. Table 1 also shows associations between IU and variables of interest. Statistically significant associations were found among PNS (
p=0.003), age (
p=0.014), number of pregnancies (
p=0.001), parity (
p=0.004), and residence location (
p=0.046). The highest proportion of IU was among women with obesity and/or aged 35 years or older and/or with at least one previous delivery and/or two or more pregnancies and/or residing in the capital city (
p<0.05). No significant differences were observed between IU and other variables analyzed in the study. The results of the multivariate analysis with the variable UI as the outcome are also presented (Table 1). It was observed that obese women (OR= 2.26; 95%CI = 1.16-4.41; p = 0.003) are more likely to use insulin to control GDM than others. On the other hand, living in the metropolitan region of Vitória proved to be a protective factor in relation to IU in controlling GDM (OR=0.61; 95%CI=0.37-0.99; p=0.046). However, this latter finding may represent a confounding factor to be discussed below.
Table 2 shows the logistic regression analysis between WG and IU according to PNS category. A statistically significant difference was found among eutrophic women (
p=0.024), with those who gained weight above the recommended level being 3.22 times more likely to use insulin compared to those who gained adequate or less-than-adequate weight.
A significant predominance of non-IU was also observed among women classified as being eutrophic or overweight (
p=0.186) who gained adequate or less-than-adequate weight (78.4 and 72%, respectively). An inversely proportional reduction of the PNS is this difference was noted in the obese women, in which percentages of insulin users and non-users were very similar to 45.6 and 43%, in which statistical significance was irrelevant (
p=0.617) for the WG variable.
For low weight, although in substantially smaller numbers, the opposite occurs when the significant predominance of IU is among those who remained with adequate or below adequate WG (75%). In this same group, the opposite occurred among those who had above adequate WG with the same percentage for non-IU (75%).
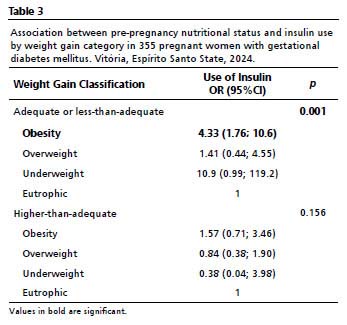
When the relationship between pre-pregnancy nutritional status and IU is analyzed for each WG category (Table 3), statistical significance is observed for the adequate or below-adequate WG group (
p=0.001). Only obese women in the study remained within the confidence interval with significantly higher chances of IU (OR= 4.33; 95%CI= 1.76-10.6;
p=0.001).
When analyzing the group with WG above the recommended level, the significance level was reduced (
p=0.156), and a negative association with the outcome variable (IU) was maintained in all PNS categories. Interestingly, the same occurs for obese women: while those with adequate or less-than-adequate WG showed significant difference, now with WG above the adequate range resembled others (OR= 1.57; 95%CI= 0.71–3.46;
p=0.156).
DiscussionBetween 70% and 85% of GDM cases can be managed with proper diet and exercise, with only 15–30% of the patients would need medication.
16 Despite this, in our study, almost half of the sample (47.04%) required adjunct insulin therapy, even in a setting where participants received multidisciplinary care including diet therapy. This finding reflects the reality that almost half of the sample consisted of women with obesity (48.7%). In addition to the 28.7% who were overweight, the result was a sample consisting of almost 80% of women who were overweight during pregnancy.
This data does not differ from the literature.
17 Eutrophic Brazilian women who receive prenatal care at the public health system represent a minority,
18 confirming the findings in the sample of this study, in which only 20.3% were classified as eutrophic. This first observation reported to us the global increase in obesity rates, which also resulted in an increase in the number of obese women who become pregnant and developed GDM two to five times more than women of normal weight compared to women with adequate weight.
19,20,21In Brazil, between 2003 and 2019, the proportion of women with obesity around 20 years of age almost doubled, with weight gain increasing proportionally with age. Among women of reproductive age, 41.4% were classified as overweight and 13.5% were obese between ages 18 and 24. These rates rose to 57% and 27.9%, respectively, among women aged 25 to 39
7an age group that comprised 83.66% of the women in this study. Age, in this study, was significantly associated with IU, as were PNS, parity, and number of pregnancies. It is important to highlight the interrelation among these variables, as older women tend to have more children and a higher BMI.
22BMI is widely recognized as the main indicator for the development of insulin resistance (IR) and GDM
9,23—as confirmed by our study, in which PNS was significantly associated with IU, with obese women using approximately twice as much insulin compared to eutrophic women. When WG was analyzed by PNS, statistical significance was observed only among eutrophic women, and with a significance reduction as BMI increased thus, following an inversely proportional pattern, in other words, the higher the BMI, the weaker the association between the WG and IU.
Among the combinations of WG by PNS and IU, the relation was found only among eutrophic women, who used considerably more insulin when they gained weight above the recommended level.
The literature has long demonstrated the role of IR as a response mechanism to weight maintenance.
24 In an attempt to maintain weight, IR increases lipolysis, resulting in the release of fatty acids and glycerol into the bloodstream, by raising blood glucose levels.
24,25 Thus, it can be inferred that the elevated serum levels of these components in cases of excessive weight may ultimately lead to a greater need for IU. Interestingly, when PNS was analyzed within each of the two WG groups, obese women who gained adequate or less-than-adequate weight had 4.22 times greater odds of IU compared to eutrophic women. However, this same association lost statistical significance among women who gained more than the adequate weight. Once again, this finding aligns with previous literature. In a randomized clinical trial, Harreiter
et al.
25 analyzed the impact of healthy eating and physical activity on maternal lipid metabolism in 436 pregnant women with obesity, aiming to promote restricted GWG. They found that, in the group with lower WG, substantially higher concentrations of free fatty acids and ketone bodies were present in the bloodstream, leading to increased blood glucose levels, particularly during fasting.
25 This suggests that pregnant women with obesity who gain insufficient weight or who lose weight may experience increased blood glucose and, consequently, a greater need for IU, which may have occurred in this study.
This issue has already been described in other studies, which shows that 50% of the pregnant women with obesity lose weight in the same proportion as they gain excessive weight.
18 Exactly as it was observed in this study, in which women with obesity showed similar percentages of adequate or less-than-adequate WG (54.4%) and higher than adequate WG (58.2%). Weight loss among pregnant women with obesity was also observed and problematized in a study involving Brazilian women that served as the basis for developing new growth curves. It is important to note that this study was conducted with women with no previous infectious or chronic conditions, except obesity, including diabetes and hypertensive disorders, and who experienced favorable pregnancy outcomes. The author describes weight loss among pregnant women with obesity as a "concerning observation, given that weight loss during pregnancy is not recommended".
12Inadequate weight gain was also observed in 545 women with GDM, 64.2%, particularly among those with obesity and overweight. IU was required in 41% of cases, and 34.8% experienced pregnancy complications, mainly hypertensive disorders.
17Recommendations for GWG are widely debated and have been the basis for systematic reviews, especially regarding women with obesity.
6,12,20,26 Bogaerts
et al.,
26 when analyzing 18,053 pregnant women with obesity, observed insufficient weight gain, including weight loss, which was associated with a reduction in gestational hypertension and emergency cesarean sections.
26 Kapadia
et al.,
6 in their review on weight gain recommendations for pregnant women with obesity, suggests that WG below the most commonly used international guidelines may, in some cases, be individually recommended based on a personalized analysis.
6These findings confirm the hypothesis that PNS can influence IU, while WG may exert its effects in different ways, depending on PNS. Excessive weight appears to be the main factor, regardless of whether it is pre-existing or acquired during pregnancy, since overweight and obese women required more IU simply due to have entered pregnancy above the recommended weight, while eutrophic women did so only after exceeding the recommended weight during pregnancy.
Indeed, pregnant women with obesity without diabetes and those with gestational diabetes without obesity demonstrate IR in similar ways, which differs from women who experience pregnancy without either condition.
27 These findings highlight the importance of further studies to support personalized approaches to GDM management, including the development of tailored WG recommendations, particularly for women with pre-existing obesity.
Specific recommendations can help optimize prenatal care, improving maternal and neonatal outcomes both in postpartum and throughout life, since GDM is already recognized as a predictor of diabetes mellitus, cardiovascular diseases, and obesity in offspring, including in adulthood.
1,2The period covered by this study (2017–2022) comprises a transitional scenario in GDM management in Brazil, with definition of diagnostic and treatment flows.
28 Diagnostic and treatment information differed among professionals and services. Therefore, it was not possible to quantify changes in glycemic control due to the lack of standardization of such information described in medical records. The UI outcome was then chosen as the most viable binary variable for quantification and/or qualification.
The study has limitations, such as its cross-sectional design, which does not allow for the assessment of causal relationships. Nevertheless, it offers relevant insights for understanding the management of gestational diabetes in different contexts of the Brazilian healthcare system. The location variable may have acted as a confounding factor, since women living in peripheral regions may have received less insulin not only because of lower clinical need, but also because of barriers to access, late diagnosis, or insufficient supplies and infrastructure.
There is also no consensus regarding the threshold or percentage of glycemic alterations required to initiate insulin therapy. The Brazilian Ministry of Health suggests that it be performed when a 30% alteration in glycemic,
29 international standards indicate lower thresholds, which reinforces the heterogeneity in conduct and the need for more precise and up-to-date national guidelines.
This study showed that both pre-pregnancy nutritional status and weight gain patterns during pregnancy influence the need for insulin treatment. It can be concluded that the treatment of gestational diabetes remains in transition, with a lack of epidemiological data in Brazil. Longitudinal studies are needed to support more assertive protocols, especially in high-risk services, as well as to inform better strategies for medication management, professionals, promotion and prevention projects in the care of GDM.
These findings reinforce the importance of specific weight gain guidelines for women with gestational diabetes, in order to support more appropriate clinical conduct and reduce maternal and fetal risks.
References1. Wahabi HA, Fayed A, Esmaeil S, Elmorshedy H, Titi MA, Amer YS,
et al. Systematic review and meta-analysis of the effectiveness of pre-pregnancy care for women with diabetes for improving maternal and perinatal outcomes. PLOS ONE. 2020 Ago; 15 (8): e0237571.
2. Mirghani Dirar A, Doupis J. Gestational diabetes from A to Z. World J Diabetes. 2017 Dez; 8 (12): 489-511.
3. Perivolaris EC, Cavalcante SKDS, Silva MNCD, Teixeira JPS, Silva VF, Dinelly ÉMP. Complicações na gravidez e diabetes mellitus na gestação: dados de morbidade e mortalidade no Brasil. Rev Soc Dev. 2021 Ago; 10 (11): e142101119335.
4. Iser BPM, Stein C, Alves LF, Carvalho MLS, Espinoza SAR, Schmidt MI. A portrait of gestational diabetes mellitus in Brazil: A systematic review and meta-analysis. Arch Endocrinol Metab. 2023 Out; 67 (6): e220521.
5. Junqueira JMO, Nascimento S, Marques SR, Fontes JF. Diabetes mellitus gestacional e suas complicações. Braz J Dev. 2021 Dez; 7 (12): 116574-89.
6. Kapadia MZ, Park CK, Beyene J, Giglia L, Maxwell C, McDonald SD. Weight Loss Instead of Weight Gain within the Guidelines in Obese Women during Pregnancy: A Systematic Review and Meta-Analyses of Maternal and Infant Outcomes. PLoS One. 2015; 10 (7): e0132650.
7. Agência Brasil 2020. IBGE: obesidade mais do que dobra na população com mais de 20 anos. [
Internet]. [access in 2024 Mar 13]. Available from:
https://agenciabrasil.ebc.com.br/saude/noticia/2020-10/ibge-obesidade-mais-do-que-dobra-na-populacao-com-mais-de-20-anos8. Knight M, Bunch K, Tuffnell D, Patel R, Shakespeare J, Kotnis R,
et al. Saving Lives, Improving Mothers' Care. 2021. [
Internet]. [access in 2024 Mar 13]. Available from:
https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/maternal-report-2021/MBRRACE-UK_Maternal_Report_2021_-_FINAL_-_WEB_VERSION.pdf9. Bolognani CV, Souza SS, Paranhos Calderon IM. Diabetes mellitus gestacional: enfoque nos novos critérios diagnósticos. Comun Ciênc Saúde. 2011; 22 (Supl. 1): S31-S42.
10. Rasmussen KM, Yaktine AL (editors); Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press; 2009. 854 p.
11. Carrilho TRB, Farias DR, Batalha MA, Costa NCF, Rasmussen KM, Reichenheim ME,
et al. Brazilian Maternal and Child Nutrition Consortium: establishment, data harmonization and basic characteristics. Sci Rep. 2020 Set; 10 (1): 14869.
12. Kac G, Carilho TRB, Rasmussen KM, Reichenheim ME, Farias DR, Hutcheon JA. Gestational weight gain charts: results from the Brazilian Maternal and Child Nutrition Consortium. Am J Clin Nutr. 2021 Mar; 113 (5): 1351-60.
13. Kusinski LC, Murphy HR, De Lucia Rolfe E, Rennie KL, Oude Griep LM, Hughes D,
et al. Dietary Intervention in Pregnant Women with Gestational Diabetes; Protocol for the DiGest Randomised Controlled Trial. Nutrients. 2020 Abr; 12 (4): 1165.
14. Zajdenverg L, Façanha C, Dualib P, Golbert A, Negrato CA, Bertoluci M. Planejamento, metas e monitorização do diabetes durante a gestação. Diretriz da Sociedade Brasileira de Diabetes (2023). [
Internet]. [access in 2023 Dez 27]. Available from:
https://diretriz.diabetes.org.br/planejamento-metas-e-monitorizacao-do-tratamento-do-diabetes-durante-a-gestacao/15. American Diabetes Association (ADA). 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes - 2024. Diabetes Care. 2024 Jan; 47 (Suppl. 1): S282-94.
16. Lende M, Rijhsinghani A. Gestational Diabetes: Overview with Emphasis on Medical Management. Int J Environ Res Public Health. 2020 Dez; 17 (24): 9573.
17. Souza ÉSS, Saunders C, Carmo CN, Aquino Lacerda EM, Zajdenverg L, Castro MBT,
et al. Gestational weight gain and adverse maternal and perinatal outcomes among women with gestational diabetes mellitus according to International Association of Diabetes and Pregnancy Study Group (IADPSG) criteria: A cross sectional study. Clin Nutr ESPEN. 2022 Ago; 50: 207-11.
18. Godoy AC, Nascimento SL, Surita FG. A systematic review and meta-analysis of gestational weight gain recommendations and related outcomes in Brazil. Clinics (São Paulo). 2015 Nov; 70 (11): 758-64.
19. Balani J, Hyer S, Johnson A, Shehata H. The importance of visceral fat mass in obese pregnant women and relation with pregnancy outcomes. Obstet Med. 2014 Mar; 7 (1): 22-5.
20. Dalfra' MG, Burlina S, Lapolla A. Weight gain during pregnancy: A narrative review on the recent evidences. Diabetes Res Clin Pract. 2022 Jun; 188: 109913.
21. Lapolla A, Dalfrà MG, Fedele D. Management of gestational diabetes mellitus. Diabetes Metab Syndr Obes. 2009 Jun; 2: 73–82.
22. Dode MASO, Santos IS. Non classical risk factors for gestational diabetes mellitus: a systematic review of the literature. Cad Saúde Pública. 2009; 25: S341-59.
23. Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. Biomedicine & Pharmacotherapy. 2021 Mai; 137: 111315.
24. Eckel RH. Insulin resistance: an adaptation for weight maintenance. Lancet. 1992 Dez; 340 (8833): 1452–3.
25. Harreiter J, Simmons D, Desoye G, Corcoy R, Adelantado JM, Devlieger R,
et al. Nutritional Lifestyle Intervention in Obese Pregnant Women, Including Lower Carbohydrate Intake, Is Associated With Increased Maternal Free Fatty Acids, 3-β-Hydroxybutyrate, and Fasting Glucose Concentrations: A Secondary Factorial Analysis of the European Multicenter, Randomized Controlled DALI Lifestyle Intervention Trial. Diabetes Care. 2019 Jun; 42 (8): 1380–9.
26. Bogaerts A, Ameye L, Martens E, Devlieger R. Weight loss in obese pregnant women and risk for adverse perinatal outcomes. Obstet Gynecol. 2015 Mar; 125 (3): 566-75.
27. Pantham P, Aye ILMH, Powell TL. Inflammation in Maternal Obesity and Gestational Diabetes Mellitus. Placenta. 2015 Jul; 36 (7): 709-15.
28. Organização Pan-Americana da Saúde/Organização Mundial da Saúde (OPAS/OMS). Ministério da Saúde (BR). Federação Brasileira das Associações de Ginecologia e Obstetrícia. Sociedade Brasileira de Diabetes. Rastreamento e diagnóstico de diabetes mellitus gestacional no Brasil. Brasília (DF): OPAS; 2017 [
Internet]. [access in 2024 Abr 19]. Available from:
https://www.febrasgo.org.br/images/pec/CNE_pdfs/Rastreamento-Diabetes.pdf29. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD,
et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar; 372: 71.
Authors' contributionVentorim PSC: Conceptualization, Data collection, curation, and analysis, Research, Methodology, Data presentation design, Writing of the original manuscript.
Souza FB: Software, Data curation and analysis, Research, Methodology, Data presentation design.
Rodrigues LK: Software, Data Curation and Analysis.
Alves DJB: Software, Data Collection and Curation.
Petarli GB: Data analysis, writing, review, and editing of the scientific text.
Cardoso LD: Writing, review, and editing of the scientific text.
Rocha JLM and Barbosa MCR: Guidance, supervision, writing, review, and editing of the scientific text.
All authors approved the final version of the article and declare no conflict of interest.
Data AvailabilityThe entire dataset supporting the results of this study was published in the article itself.
Received on May 27, 2024
Final version presented on September 1, 2025
Approved on September 10, 2025
Associated Editor: Karla Bomfim
 ; Fernando Barbosa de Souza3
; Fernando Barbosa de Souza3 ; Letícia Karina Rodrigues2
; Letícia Karina Rodrigues2 ; Débora Jandira Bruschi Alves3
; Débora Jandira Bruschi Alves3 ; Glenda Blaser Petarli2
; Glenda Blaser Petarli2 ; Luciane Daniele Cardoso3
; Luciane Daniele Cardoso3 ; José Luiz Marques-Rocha1
; José Luiz Marques-Rocha1 ; Míriam Carmo Rodrigues Barbosa1
; Míriam Carmo Rodrigues Barbosa1