ABSTRACT
OBJECTIVES: to analyze growth velocity trajectories according to exclusive breastfeeding practices among preterm and low-birth-weight infants followed within the Kangaroo Mother Care.
METHODS: a longitudinal study was conducted with 152 newborns cared for in two public maternity hospitals in Fortaleza, Ceará, Brazil, between October 2016 and October 2017. The exposure variable was exclusive breastfeeding at Kangaroo Mother Care discharge. Weight gain (g/kg/day), length (cm/day), and head circumference (cm/day) velocities were estimated from repeated anthropometric measurements using linear mixed-effects models with restricted cubic splines, adjusted for neonatal and maternal characteristics.
RESULTS: overall, 51.3% of the infants were female, with a mean birth weight of 1,743 g (±43.8) and a mean gestational age of 32.7 weeks (±2.1). At 37 weeks of age, exclusively breastfed infants showed higher weight gain velocity (11.8 g/kg/day; 95%CI= 10.7-12.9) compared with those not exclusively breastfed (8.4 g/kg/day; 95%CI= 6.4-10.5; p<0.05). No statistically significant differences were observed in length or head circumference trajectories between feeding groups.
CONCLUSIONS: exclusive breastfeeding at Kangaroo Mother Care discharge was associated with higher weight gain velocity at 37 weeks of life, underscoring the importance of breastfeeding in promoting healthy growth among preterm and low-birth-weight infants.
Keywords:
Exclusive breastfeeding, Growth, Preterm infant, Low birth weight infant, Kangaroo-mother care
RESUMO
OBJETIVOS: analisar as trajetórias de velocidade de crescimento segundo a prática de aleitamento materno exclusivo em recém-nascidos prematuros e de baixo peso acompanhados pelo Método Canguru.
MÉTODOS: estudo longitudinal com 152 recém-nascidos acompanhados em duas maternidades públicas de Fortaleza/Ceará, entre outubro de 2016 e outubro de 2017. A variável de exposição foi o aleitamento materno exclusivo na alta do Método Canguru. Velocidades de ganho de peso (g/kg/dia), comprimento (cm/dia) e perímetro cefálico (cm/dia) foram estimadas a partir de medidas antropométricas repetidas, utilizando modelos lineares mistos com splines cúbicas restritas, ajustados para características neonatais e maternas.
RESULTADOS: do total, 51,3% eram do sexo feminino, com peso médio ao nascer de 1.743 g (±43,8) e idade gestacional de 32,7 semanas (±2,1). Às 37 semanas, os lactentes em aleitamento materno exclusivo apresentaram maior velocidade de ganho de peso (11,8 g/kg/dia; IC95%= 10,7–12,9) em comparação aos não exclusivamente amamentados (8,4 g/kg/dia; IC95%=6,4–10,5; p<0,05). Não foram identificadas diferenças estatisticamente significativas nas trajetórias de comprimento e perímetro cefálico entre os grupos de aleitamento.
CONCLUSÃO: o aleitamento materno exclusivo até a alta do Método Canguru foi associado a maior velocidade de ganho de peso às 37 semanas de vida, reforçando a relevância da amamentação na promoção do crescimento saudável de recém-nascidos prematuros e de baixo peso.
Palavras-chave:
Aleitamento materno exclusivo, Crescimento, Recém-nascido prematuro, Recém-nascido de baixo-peso, Método canguru
IntroductionPrematurity (childbirth preceding 37 gestational weeks) and low birth weight (LBW, weight <2,500g) are major causes of neonatal mortality.
1 The risk of neonatal death is 16 times greater for preterm newborns (NB) compared to those born at term, and 25 times greater for those with LBW compared to those born with adequate weight.
2Preterm newborns and those with LBW accounted for 9.5% and 9.6%, respectively, of births that occurred in Brazil between 2011 and 2018.
2 This scenario represents an ongoing challenge for health services, particularly concerning postnatal growth. Factors such as metabolic and gastrointestinal immaturity, higher susceptibility to infections and adverse perinatal conditions, such as intrauterine growth restriction and respiratory complications, compromise growth and long-term health outcomes.
3,4 Moreover, unfavorable socioeconomic aspects can further exacerbate extrauterine growth deficit and impair its recovery.
4Exclusive breastfeeding (EBF) is among the key factors that promote postnatal growth, and it is known for its benefits in weight gain, infant development, and infection control.
5 In this regard, Kangaroo Mother Care (KMC), which the World Health Organization (WHO) recommends as a humanized perinatal care model for preterm and low birth weight (LBW) newborns, is considered a vital strategy for fostering healthy growth and breastfeeding in this population.
6A systematic review with meta-analysis, which included 32 randomized clinical trials conducted in low-, middle-, and high-income countries, revealed consistent benefits of KMC for growth and breastfeeding. Infants followed up in KMC showed greater weight gain (mean difference (MD) of 4.08g/day, 95% confidence interval [95%CI] 2.30, 5.86), length (MD of 0.21cm/week, 95%CI= 0.03, 0.38) and head circumference (HC) (MD of 0.18cm/week, 95%CI=0.09, 0.27), compared to those who received conventional care. Furthermore, a higher probability of EBF was observed at discharge or at 28 days of life (relative risk [RR] 1.48, 95% CI = 1.44-1.52) among infants followed in the KMC group.
7The longitudinal follow- up of growth in preterm and LBW newborns is essential for comprehending postnatal development and improving long-term health outcomes. Given that infant growth is non-linear, the growth velocity can identify variations such as periods of acceleration and stagnation,
8 providing a more consistent assessment. A Chinese population-based cohort of 1,221 preterm newborns identified, for example, faster velocities of weight and length gain between the third and sixth months of life.
9The growth monitoring via KMC enables the early identification of nutritional deviations. However, regional evidence from vulnerable socioeconomic contexts does not detail the evolution of growth according to feeding practices, such as EBF.
10 Investigating this dynamic can optimize nutritional and health strategies for this population.
Accordingly, this study aimed to analyze trajectories of growth velocity according to the practice of EBF in preterm and LBW infants followed in KMC.
MethodsThis was a longitudinal study that included preterm and LBW newborns followed in KMC at two Brazilian maternity hospitals located in Fortaleza, Ceará. These facilities are among the first reference centers that actively participated in the process of implementation and scaling up the KMC in Brazil.
11KMC was carried out in three stages, according to Brazilian Ministry of Health Ordinance N
o. 1,683, of July 12, 2007.
12 The first stage took place in the Neonatal Intensive Care Unit (NICU), where parents were encouraged to practice skin-to-skin contact based on the infant's clinical stability. The second stage, after discharge from the NICU, was conducted in the Kangaroo Intermediate Care Unit (KICU), where mothers and infants were encouraged to maintain the kangaroo position (newborn in skin-to-skin contact, wearing only a diaper in a vertical position against the mother's chest) for as long as both found it comfortable and adequate. In the third stage, after hospital discharge, the newborns continued with outpatient follow-up to monitor weight gain and guide the family on home care, with a number of appointments varying from one to six per newborn.
Data collection started during the third stage of KMC to reduce loss to follow-up. Information from previous stages was obtained retrospectively from medical records and service documents, while data from the third stages was collected prospectively through interviews with mothers during outpatient appointments. The sample collected between October 2016 and October 2017, included preterm and LBW newborns who attended the first appointment of the third stage, and whose mothers agreed to participate in the study. Children with malformations, chronic diseases, or who did not complete all KMC appointments were excluded.
The sample size calculation was estimated based on parameters from the literature concerning postnatal growth in preterm newborns.
9 Considering minimum clinically relevant differences between groups, α = 5% and 80% power, the minimum estimated sample sizes ranged from 46 to 68 newborns. The final sample (n=152) was adequate to detect differences between EBF groups (yes/no) (Figure 1), with each maternity hospital contributing 62 and 90 newborns, respectively.
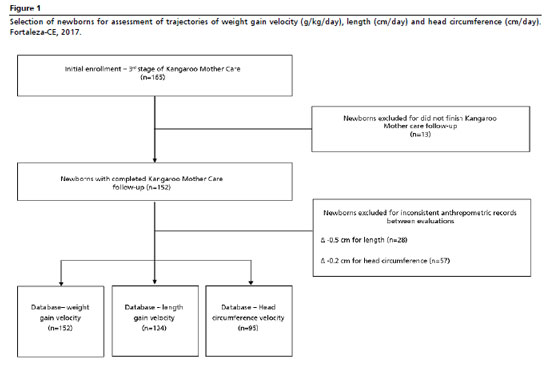
The practice of EBF at KMC discharge was defined as the independent variable, determined after the repeated anthropometric measures. It was classified as yes (newborns who remained exclusively breastfed throughout the three KMC stages) and no (newborns who discontinued EBF before KMC discharge). In the third stage, at each appointment, mothers reported the provision of breast milk, as well as other liquids such as water, tea, formulas, cow's milk, and food. Data on newborn characteristics (sex, birth weight, and gestational age) and maternal characteristics (age, educational attainment, income and pre-gestational BMI) were collected during the initial interview.
According to standardized protocols for collecting anthropometric data in health services,
13 the measurements of weight, length and HC were collected at birth, at admission to and discharge from the first two KMC stages, as well as at admission to and during scheduled follow-up appointments of the third stage until final discharge. Weight was obtained using a digital pediatric scale (15 kg capacity, 10 g accuracy), with the infant unclothed. Length was measured with a wooden infantometer (0-100 cm range, 1mm accuracy), and HC with a non-stretch measuring tape (1 mm accuracy).
13Based on anthropometric data, the growth velocities for weight gain (g/kg/day), length (cm/day) and HC (cm/day) were calculated using the formulas described below.
14.15 Velocities were calculated across the following intervals: birth, discharge from the first KMC stage, discharge from the second KMC stage, admission to the third KMC stage, and subsequent appointments during the third stage until final discharge from the method.
Weight gain velocity (g/kg/day) = [final weight (g) – initial weight (g)]/ [(mean weight (kg)/ number of days]. The mean weight (kg) corresponds to the average of the initial and final weight for each specified interval.
Length and HC gain velocity (cm/day) = [final measurement (cm) - initial measurement (cm)/ number of days].
Covariates included infant sex (male/female), birth weight (<1,500; ≥1,500 g), gestational age at birth (<32; ≥32 gestational weeks). Maternal variables considered age (< 19, 20-34, ≥35 years), educational attainment (< 9; ≥9 years of schooling), family income (<2, ≥2 minimum wages), presence of a partner (yes/no), residence (capital and metropolitan area; rural area) and pre-gestational overweight/obesity (yes/no, defined as body mass index ≥25kg/m
2).
For data analysis, continuous variables were described by the mean and standard deviation (SD), considering their normal distribution which was verified using the Shapiro-Wilk test in conjunction with histogram inspection. The EBF distribution (yes/no) at KMC discharge was compared based on infant and maternal characteristics using the chi-square test. Trajectories of weight gain, length and HC velocities according to EBF at KMC discharge were estimated using linear mixed models with restricted cubic splines. This approach is well-suited for unbalanced longitudinal data, where the number and timing of measurements vary across individuals.
16 Cubic splines, composed of piecewise polynomials, smooth the linear relationship between anthropometric measurements and age, thereby capturing the non-linear structure of infant growth.
17Piecewise polynomials were united in knots and placed in the ages of 32, 36, 40 and 44, as these represent critical growth milestones in preterm infants. Each outcome was assessed in specific subgroups, based on measurement quality criteria. Newborns with negative variations greater than -0.5 cm in length and -0.2 cm in HC were excluded, considering biological implausibility of these reductions and the limits of inter-rater standard error.
18 Ultimately, 152 children were followed for weight, 124 for length and 95 for HC (Figure1).
Initially, the models included the outcome of interest (velocity of weight gain, length or HC), the EBF exposure, infant's sex, linear and spline terms for the child's age in weeks and interaction terms for EBF and age. Random effects for the intercept and linear term for age (slope) were included into account for the intrapersonal correlation of measurements in the variance estimation.
19 The final models were adjusted, based on their conceptual relevance for fetal growth, for the following covariates: maternal educational, family income, maternal age, presence of a partner, area of residence, pre-gestational overweight/obesity, gestational age, and birth weight. Models including the total duration of KMC were also tested, but did not alter the results. Ultimately, the selected adjusted model was the one that presented the lowest values for the Akaike Information Criterion and the Bayesian Information Criterion,
20 without the inclusion of the total duration of KMC. The predicted values of weight gain, length and HC velocities and their 95% CIs were estimated weekly, considering the newborns' age range, according to EBF. All analyses were performed using Stata software, version 17.
The research was approved by the Research Ethic Committees of the two participating maternity hospitals (approval numbers 1882420 and 1.551.528).
ResultsMore than half (51.3%) of the newborns were female, with a mean (±SD) birth weight of 1743.4 (±43.8) g and a mean gestational age at birth of 32.7 (±2.1) weeks. The mean follow-up time in the KMC was 43.0 (±18.2) days. During this period, the newborns contributed 619 measurements for weight, 500 for length, and 373 for HC, ranging from two to eight measurements per newborn. The mean velocities (±SD) were 8.47 (±10.61) g/kg/day, 0.13 (±0.14) cm/day, and 0.11 (±0.09) cm/day for weight, length, and HC, respectively.
According to Table 1, no statistically significant differences were observed between the newborn groups that maintained EBF until KMC discharge, and those who did not, based on infant and maternal characteristics. (Table 1).
In the initial model, higher mean weight gain velocities were observed between 36 and 38 weeks of age among newborns maintained on EBF throughout the KMC. In the adjusted model, the weight gain velocity was negative until 33 weeks of age. A similar pattern was observed, with newborns on EBF showing a higher mean weight gain velocity, but only at 37 weeks of life (11.83 g/kg/day, 95% CI = 10.67–12.99), compared to newborns not exclusively breastfed until KMC discharge (8.42 g/kg/day, 95% CI = 6.38–10.45) (Table 2, Figure 2).
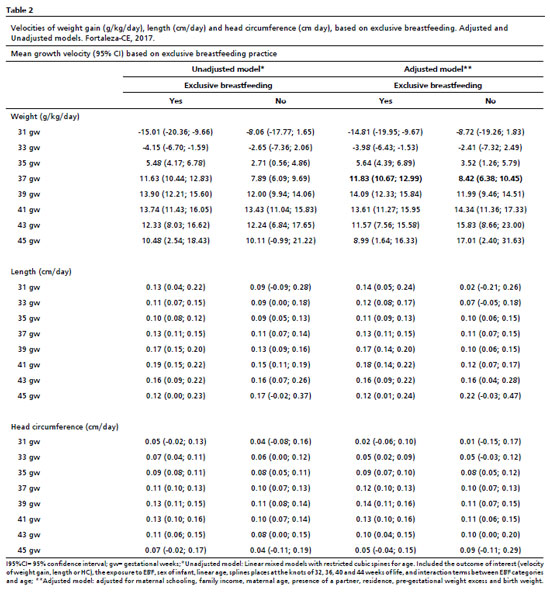
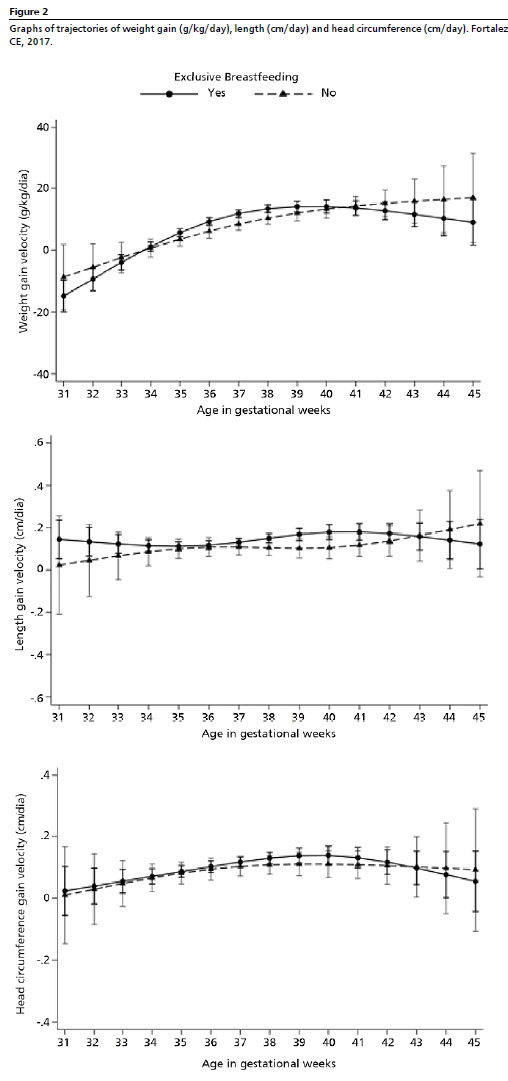
Regarding weight length velocity, both the adjusted and unadjusted models demonstrated that children on EBF had higher means between the 40 and 41 weeks of age. Among those not exclusively breastfed, the velocity peak occurred around the 46th week (Table 2, Figure 2). However, no statistically significant differences were identified in growth trajectories between newborns on EBF and those not exclusively breastfed. Concerning HC growth velocity, the highest means were recorded between the 38 and 39 weeks of age, similar across both models and in both feeding groups. Likewise, no statistically significant differences were observed in HC growth trajectories according to the EBF practice (Table 2, Figure 2).
DiscussionThis study explored the influence of EBF on the trajectories of weight gain, length, and HC velocities in preterm and LBW newborns followed in KMC. The findings showed that newborns exclusively breastfed throughout the KMC period achieved a significantly higher mean weight gain velocity at 37 weeks of life. No differences were found in trajectories of length and HC growth between the breastfeeding groups during the study period.
Recommendations for assessing preterm newborn growth set targets for weight gain velocity (15g/kg/day), length (1cm/week), and HC (0.5-1cm/week).
9 In the present study, although the mean observed velocities were lower for weight and length, the results should be interpreted by considering the specific nature of preterm growth and the duration of follow-up within KMC.
The studied newborns experienced weight loss until 33 weeks of life, which may be explained by physiological and clinical factors characteristic to prematurity. Immediately after birth, preterm neonates are more susceptible to significant weight loss, compared to term neonates, due to immaturity of the cutaneous barrier, respiratory function, leading to the increase of insensible water loss, also renal function.
21 Furthermore, the slower advancement of enteral feeding, associated with clinical complications and the need for fasting for examinations, can result in negative energy and protein balance, thereby contributing to weight loss in the early weeks of life.
22Subsequently, growth rates remained lower for preterm infants until approximately three months of corrected age, as identified in a Chinese birth cohort with 198 matched preterm and term infants.
23 In this study, EBF until discharge from KMC was associated with a greater weight gain velocity at 37 weeks of age. Although this effect was not maintained in the subsequent weeks, it may indicate the onset of nutritional recovery of newborns, particularly among those who received adequate support through EBF.
Although evidence suggests that formula-fed preterm and low birth weight infants grow faster than those exclusively breastfed,
24 these gains predominantly reflect the accumulation of fat mass, which, in the long term, is associated with poorer metabolic outcomes. A metanalysis of six studies from high-income countries demonstrated that formula-fed preterm infants fed had greater fat mass at term (mean difference, 0.24 kg; 95%CI = 0.17-0.31) and a higher fat percentage at 36 weeks (mean difference, 3.70%; 95%CI=1.81-5.59) compared to breastfed infants.
25Human milk appears to be the optimal choice for these infants, as it promotes a more appropriate body composition, with greater fat-free mass deposition,
26 providing a more physiological growth trajectory in the long term. From this perspective, the so-called "breastfeeding paradox" hypothesizes that human milk intake, despite promoting a slower initial weight gain, is associated with benefits such as increased fat-free mass accretion and better neurocognitive development in preterm infants.
27-28Accordingly, sustaining EBF within the KMC context is a core strategy for promoting healthy growth in high-risk neonates, as recommended by the WHO.
29 KMC facilitates effective mothers-infant bonding and maternal engagement in care, including breastfeeding, making mothers feel more secure and empowered in their caregiving role, thereby increasing the likelihood of continued EBF.
30In this study, no effects of EBF were observed on linear growth and HC, possibly because these parameters are less responsive to short-term nutritional interventions, unlike weight.
The limitations include a small sample size, which may have limited the power to detect differences, and the relatively short follow-up period, which may have underestimated the effects on later growth outcomes. The measurement of EBF also may be susceptible to information bias, and the grouping of children that discontinued the practice at varying durations may have attenuated the associations. Although the data are from 2017, KMC practices and the neonatal growth standards remain current, which supports the external validity of the results.
As strengths, this study achieved the collection of data from three KMC stages, which represents a key differential, given the high rates of loss to follow-up and discontinuity observed in similar studies. Furthermore, it provided regional longitudinal evidence on the association between EBF and weight gain in preterm infants, particularly in vulnerable socioeconomic contexts.
In conclusion, EBF was associated with a greater weight gain velocity at 37 weeks of life, which reinforces its relevance in promoting healthy growth among high-risk neonates. Further studies, with longer follow-up durations, are needed to clarify its effects across different dimensions of growth.
References1. Bernardino FBS, Gonçalves TM, Pereira TID, Xavier JS, Freitas BHBM, Gaíva MAM. Tendência da mortalidade neonatal no Brasil de 2007 a 2017. Ciênc Saúde Colet. 2022; 27 (2): 567-78.
2. Paixao ES, Blencowe H, Falcao IR, Ohuma EO, Rocha ADS, Alves FJO,
et al. Risk of mortality for small newborns in Brazil, 2011-2018: A national birth cohort study of 17.6 million records from routine register-based linked data. Lancet Reg Health Am. 2021; 3: 100045.
3. Jańczewska I, Wierzba J, Jańczewska A, Szczurek-Gierczak M, Domżalska-Popadiuk I. Prematurity and Low Birth Weight and Their Impact on Childhood Growth Patterns and the Risk of Long-Term Cardiovascular Sequelae. Children (Basel). 2023; 10 (10): 1599.
4. Ravi K, Young A, Beattie RM, Johnson MJ. Socioeconomic disparities in the postnatal growth of preterm infants: a systematic review. Pediatr Res. 2025; 97 (2): 532-57.
5. Juharji H, Albalawi K, Aldwaighri M, Almalki A, Alshiti H, Kattan W,
et al. Impact of Breastfeeding on Low Birthweight Infants, Weight Disorders in Infants, and Child Development. Cureus. 2022; 14 (12): e32894.
6. Ministério da Saúde (BR). Secretaria de Atenção Primária à Saúde. Departamento de Ações Programáticas Estratégicas. Método canguru: diretrizes do cuidado. 1ª ed. rev. [
Internet]. Brasília (DF): Ministério da Saúde; 2018. [access in 2025 Mar 10]. Available from:
https://bvsms.saude.gov.br/bvs/publicacoes/metodo_canguru_diretrizes_cuidado_revisada.pdf 7. Sivanandan S, Sankar MJ. Kangaroo mother care for preterm or low birth weight infants: a systematic review and meta-analysis. BMJ Glob Health. 2023; 8 (6): e010728.
8. Greer FR, Olsen IE. How fast should the preterm infant grow? Curr Pediatr Rep. 2013; 1: 240–6.
9. Kang L, Wang H, He C, Wang K, Miao L, Li Q,
et al. Postnatal growth in preterm infants during the first year of life: A population-based cohort study in China. PLoS One. 2019;14 (4): e0213762.
10. Andrade L, Kozhumam AS, Rocha TAH, de Almeida DG, Silva NC, Souza Queiroz RC,
et al. Impact of socioeconomic factors and health determinants on preterm birth in Brazil: a register-based study. BMC Pregnancy Childbirth. 2022; 22 (1): 872.
11. Ministério da Saúde (BR). Secretaria de Atenção à Saúde. Departamento de Ações Programáticas e Estratégicas. Atenção humanizada ao recém-nascido de baixo peso: Método Canguru. 2
nd ed. 1. reimpr. Brasília (DF): Ministério da Saúde; 2013. [access in 2025 Mar 10]. Available from:
http://bvsms.saude.gov.br/bvs/publicacoes/atencao_humanizada_recem_nascido_canguru.pdf 12. Brasil. Portaria n° 1.683/GM, 12 de julho de 2007. Aprova, na forma de anexo, a Norma de orientação para a implantação do Método Canguru. Diário Oficial da União. Brasília (DF): 12 de julho de 2007. [access in 2025 Mar 10]. Available from:
https://bvsms.saude.gov.br/bvs/saudelegis/gm/2007/prt1683_12_07_2007.html 13. Ministério da Saúde (BR). Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Orientações para a coleta e análise de dados antropométricos em serviços de saúde: Norma técnica do Sistema de Vigilância Alimentar e Nutricional – SISVAN.
Brasília (DF): Ministério da Saúde; 2011. [access in 2025 Fev 15]. Available from:
http://189.28.128.100/nutricao/docs/geral/orientacoes_coleta_analise_dados_antropometricos.pdf 14. Fenton TR, Chan HT, Madhu A, Griffin IJ, Hoyos A, Ziegler EE,
et al. Preterm Infant Growth Velocity Calculations: A Systematic Review. Pediatrics. 2017; 139 (3): e20162045.
15. Fenton TR, Nasser R, Eliasziw M, Kim JH, Bilan D, Sauve R. Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant. BMC Pediatr. 2013; 13: 92.
16. Fausto MA, Carneiro M, Antunes CM de F, Pinto JA, Colosimo EA. O modelo de regressão linear misto para dados longitudinais: uma aplicação na análise de dados antropométricos desbalanceados. Cad Saúde Pública. 2008; 24 (3): 513-24.
17. Howe LD, Tilling K, Matijasevich A, Petherick ES, Santos AC, Fairley L,
et al. Linear spline multilevel models for summarising childhood growth trajectories: A guide to their application using examples from five birth cohorts. Stat Methods Med Res. 2016; 25 (5): 1854-74.
18. World Health Organization (WHO). Training course on child growth assessment. Module B: Measuring a child's length and height. Geneva: WHO; 2008. [access in 2025 Fev 15]. Available from:
https://iris.who.int/bitstream/handle/10665/43601/9789241595070_H_eng.pdf?sequence=8&isAllowed=y19. Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of longitudinal data. 2
nd ed. Oxford: Oxford University Press; 2002.
20. Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. 2
nd ed. New York: Springer; 2002.
21. Valentine GC, Umoren RA, Perez KM. Early inadequate or excessive weight loss: A potential contributor to mortality in premature newborns in resource-scarce settings?. Pediatr Neonatol. 2021; 62 (3): 237-9.
22. Lygerou I, Ilia S, Briassoulis P, Manousaki A, Koropouli M, Hatzidaki E,
et al. The Impact of Estimated Energy and Protein Balances on Extrauterine Growth in Preterm Infants. Nutrients. 2023 Aug 11; 15 (16): 3556.
23. Zhonggui X, Ping Z, Jian K, Feimin S, Zeyuan X. The growth rates and influencing factors of preterm and full-term infants: A birth cohort study. Medicine (Baltimore). 2022; 101 (34): e30262.
24. Lu AS, Harrison CM. Formula feeding results in better growth and weight gain compared to donor breast milk in preterm and low birthweight infants, with a greater risk in necrotising enterocolitis. Arch Dis Child Educ Pract Ed
. 2020; 105 (6): 381-2.
25. Huang P, Zhou J, Yin Y, Jing W, Luo B, Wang J. Effects of breast-feeding compared with formula-feeding on preterm infant body composition: a systematic review and meta-analysis. Br J Nutr. 2016; 116 (1): 132-41.
26. Cerasani J, Ceroni F, De Cosmi V, Mazzocchi A, Morniroli D, Roggero P,
et al. Human Milk Feeding and Preterm Infants' Growth and Body Composition: A Literature Review. Nutrients. 2020; 12 (4): 1155.
27. Giannì ML, Consales A, Morniroli D, Vizzari G, Mosca F. The "Breastfeeding Paradox" as a Guide for the Assessment of Premature Infants Growth: It Is More Than Just Weigh-Ins. Breastfeed Med. 2023; 18 (5): 385-7.
28. Machado DR, Silva MC. The influence of exclusive breastfeeding on preterm newborns and development. Res Soc Dev. 2023; 12 (13): e29121344115.
29. Care of Preterm or Low Birthweight Infants Group. New World Health Organization recommendations for care of preterm or low birth weight infants: health policy. EClinicalMedicine. 2023 Ago; 63: 102155.
30. Alves FN, Azevedo VMGO, Moura MRS, Ferreira DMLM, Araújo CGA, Mendes-Rodrigues C,
et al. Impacto do método canguru sobre o aleitamento materno de recém-nascidos pré-termo no Brasil: uma revisão integrativa. Ciênc saúde coletiva. 2020; 25(11): 4509–20.
AcknowledgementsWe thank the
Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP) for the financial support – Project number FUNCAP 0898/2015. Process: 88887.100545/2015.
Author's contributionSalviano AF: conceptualization, data analysis and interpretation, manuscript writing
Azevedo LHB and Maneschy IR: data interpretation and critical review of the manuscript.
Almeida PC and Azevedo DV: conceptualization and study design, analysis supervision, data interpretation and critical review of the manuscript.
All authors approved the final version of the article and declared no conflicts of interest.
Data availabilityAll datasets supporting the results of this study are included in the article.
Received on July 2, 2025
Final version presented on September 14, 2025
Approved on September 16, 2025
Associated Editor: Paola Mosquera
 ; Lídia Helena Bezerra Azevedo2
; Lídia Helena Bezerra Azevedo2 ; Ivie Reis Maneschy3
; Ivie Reis Maneschy3 ; Paulo César de Almeida4
; Paulo César de Almeida4 ; Daniela Vasconcelos de Azevedo5
; Daniela Vasconcelos de Azevedo5
