ABSTRACT
OBJECTIVES: to evaluate the incidence and factors associated with bronchopulmonary dysplasia (BPD) in premature infantsdischarged from Neonatal Intensive Care Units (NICU).
METHODS: retrospective cohort study with premature infants discharged from the NICU. The sampling process was random, considering premature infants being followed up at a high-risk outpatient clinic registered in a NICU from 2014 to 2018. The collection was carried out from medical records and interviews with mothers or guardians, with information regarding complications during pregnancy, conditions of birth, care and morbidities in the NICU. BPD was measured for premature neonates using oxygen therapy for a period equal to or greater than 28 days or 36 weeks of corrected gestational age. After bivariate analyses, binary logistic regression analysis was followed. For the final model, a significance level of 5% (p<0.05) was defined, with registration of the respective Odds Ratio and 95% confidence intervals.
RESULTS: data from 293 preterm infants, predominantly male (55.6%) with gestational age from 32 to 36 weeks, were evaluated. BPD was recorded for 63 children (21.5%). The variables that remained statistically associated with BPD were: birth weight, gestational age and late sepsis.
CONCLUSIONS: there was a high incidence of BPD. Associated factors highlight the need for improving pre- and postnatal care.
Keywords:
Bronchopulmonary dysplasia, Premature birth, Risk factors
RESUMO
OBJETIVOS: avaliar a incidência e fatores associados à displasia broncopulmonar (DBP) em egressos de Unidades de Terapia Intensiva Neonatal (UTIN).
MÉTODOS: estudo de coorte retrospectivo com prematuros egressos de UTIN. O processo amostral foi aleatório, considerando crianças em seguimento em ambulatório de alto risco com registro em UTIN no período de 2014 a 2018. A coleta foi realizada a partir de prontuários e entrevistas com as mães ou responsáveis, com informações referentes às intercorrências durante a gestação, condições de nascimento, cuidados e morbidades na UTIN. A DBP foi aferida para neonatos com utilização de oxigenioterapia por períodoigual ou superior a 28 dias ou 36 semanas de idade gestacional corrigida. Após análises bivariadas, seguiu-se análise de regressão logística binária. Para o modelo final definiu-se nível de significância de 5% (p<0,05), com registro das respectivas Odds Ratio e intervalos de confiança de 95%.
RESULTADOS: foram avaliados dados de 293 prematuros, predominantemente do sexo masculino (55,6%) e idade gestacional de 32 a 36 semanas. A DBP foi registrada para 63 crianças (21,5%). As variáveis que permaneceram estatisticamente associadas à DBP foram: peso de nascimento, idade gestacional e registro de sepse tardia.
CONCLUSÕES: registrou-se elevada incidência de DBP. Os fatores associados destacam a necessidade de melhoria dos cuidados pré e pós-natais
Palavras-chave:
Displasia broncopulmonar, Nascimento prematuro, Fatores de risco
IntroductionIn recent years, improvements in perinatal and neonatal care have assured the survival of increasingly preterm newborns (NB). Worldwide, it is estimated that 13.4 million newborns were born prematurely (<37 weeks) in 2020, which represents 10% of all births. Over the half of these births occurred in developing countries.
1 The Neonatal Intensive Care Units (NICU) have registered positive results, although the circumstances involved in the survival of the very preterm NB also imply in risks of sequels that may compromise the quality of life of both children and their families.
2Bronchopulmonary dysplasia (BPD) is one of the main morbidities that affect premature NBs. It is a clinical condition of complex etiology that involves bronchial epithelium hyperplasia of the developing lungs.
3,4 It is described as a condition with distinct risk factors and a record of anincrease of itsoccurrence, mainly in extreme preterm newborns
4. The authors of a recent systematic review, published in 2021, reported that the informed global incidence of bronchopulmonary hyperplasia among preterm newborns varied from 10% to 89%, with values that reached percentages of 73% in Europe, 89% in North America, 82% in Asia and 62% in Oceania.
5In the last two decades, BPD is recognized as a disease not restricted only to the neonatal period and that may increase the risk of retinopathy of prematurity and neurological alterations. There are other morbidities also associated with BPD, which may be present even in older children, such as diminished pulmonary function, increase in the incidence of emphysema and higher risk of wheezing. In addition, there are extrapulmonary complications, such as increased cardiovascular risk and higher incidence of arterial hypertension in adolescence and adulthood.
6,7The literature indicates that predictors or factors associated with BPD are diverse and multifactorial and may be related to both prenatal and postnatal periods, including: chorioamnionitis, intrauterine growth restriction, low gestational age, low birth weight, male gender, patent ductus arteriosus, necrotizing enterocolitis, late-onset sepsis, need for positive mechanical ventilation, need for supplementary oxygen and increase of the fluid intake in the first days of life.
3,4,6-8 BPD occurrence is still significant and have been increasing, standing out as a critical condition for family care and health services.
5There are scarce national recent studies concerning BPD. One of the difficulties for the development of surveys regarding this subject is the dilemma on BPD definition itself. A consensual definition presented collectively by three important agencies from the United States - the National Institute of Child Health and Human Development;the National Heart, Lung, and Blood Institute and the NIH Office of Rare Diseases, in the year 2000, has predominated and highlights a functional conceptualization for BPD considering the need for supplemental oxygen therapy by preterm neonates for a period equal or higher than 28 days or 36 weeks of corrected gestational age.
9,10It is relevant to understand all aspects associated with BPD and thus enabling opportune interventions. In this regard, regional surveys may be useful, guiding quickly adaptable measures and interventions that are more effective. We did not find any study concerning this subject for the North region of Minas Gerais. It is a region with a large territorial extension (higher than many Brazilian states) whose most complex healthcare facilities are focused in a single city. The region is one of the poorest in the country, considering its socioeconomic indicators and still has a lack of technical and human resources for mother-and-child care. The only outpatient clinic for preterm children and children discharged from NICU is located in Montes Claros, the region’s hub city. In this perspective, this study aimed to assess the incidence and factors associated with bronchopulmonary dysplasia in children discharged from NICUs in the North of Minas Gerais.
MethodsA retrospective cohort studycarried out in a follow-up outpatient clinic for high-risk newborns that attend newborns discharged from NICUs, in Montes Claros (MG). The city is the main urban hub of the North region of Minas Gerais and has a population of approximately 415,000 inhabitants.
11 With regard to the primary care services, the city has full coverage, with a record of over 150 teams of Family Health Strategy, which are responsible for the identification and follow-up of habitual risk pregnancy. The high-risk pregnancies are followed-up in a specialized center for women’s health assistance. There are three great maternity hospitals in the city with NICU services, and the North region has other two NICUs in satellite cities. The newborns are entered in the follow-up outpatient clinic, after NICU discharge, following the detailed medical referral of birth conditions and history of hospital stay, and are assisted by a multiprofessional team.
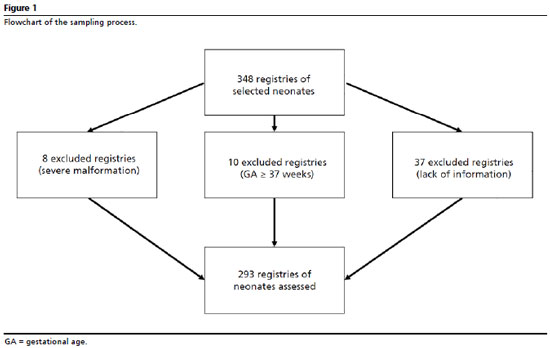
The study population was built with preterm newborns (gestational age registered in the medical record less than 37 weeks). The sampling process was random, considering neonates hospitalized in NICUs in the period from March 2014 to July 2018, regardless of the birth weight. Children who had severe malformations were excluded. Children whose medical records had lacking information and the mothers/guardians could not inform were considered losses. For the sample size determination, it was estimated a 95% confidence interval and 80% power, estimated relation non-exposed/exposed and Relative Risk equal to three, which defined a minimum sample size of 290 children. These values were considered based on similar studies, observing the absence of previous local studies.
7,12-15 Considering potential losses, the number of estimated medical records was increased 20%. For the random sampling process, from the sequence of numbers of medical records registered each month, six to eight medical records were raffled for each month in the period considered in the research. The flowchart presented in Figure 1 details the allocation of losses of the process. Data collection was conducted by a specifically trained team with the use of a form developed for this study. Data were collected from analyzes of medical records of registered children after NICU discharge and from interviews executed with mothers or guardians.
Information concerning intercurrences during pregnancy (maternal hypertension, maternal diabetes, maternal urinary tract infection during pregnancy and use of antenatal corticosteroid), birth conditions (type of delivery, gender of neonate, birth weight, gestational age and need for resuscitation in birth room), care and intercurrences/morbidities registered during NICU stay (early and late onset sepsis, congenital cardiomyopathy, need for blood transfusion,as a marker for severe anemia and use of vasoactive amines, as a marker for severe hemodynamic instability and time of mechanical ventilation). The answer variable was the registry of BPD. The definition of BPD was measured, following the aforementioned references,
9.10 It was not possible to classify BPD due to the lack of registration in some records about the fraction of inspired oxygen (FiO
2).
For data processing, we used the IBM-SPSS for Windows version 22.0 software. The frequency distribution of main variables and bivariate analysis for identification of variables associated with BDP was performed. Those who were associated up to the level of 20% (
p<0.20) were assessed jointly by means of binary logistic regression. For the final model, only variables associated up to the significance level of 5% (
p<0.05%) were maintained, with registration of the respective odds ratio and 95% confidence intervals.
The research was conducted with the proper respect to every ethical aspects and the research project was approved by the Research Ethics Committee of the institution that houses the study (Federal University of Monte Claros – MG), under the opinion number 1.800.915 and CAAE 58699016.8.0000.5146.
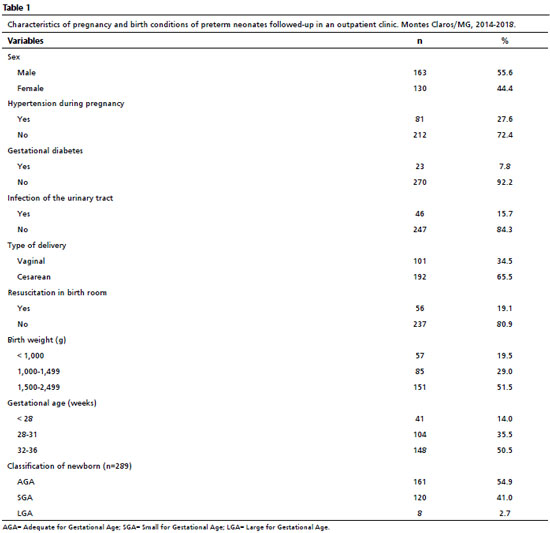
ResultsThe number of preterm newborns discharged from NICU and who participated in the study was 293, and they were followed-up at the outpatient clinic for high-risk newborns. Of these, 163 (55.6%) were male and 57 (19.5%) had birth weight inferior to 1000 grams. The group assessed was composed mainly by moderate preterm newborns, that is, those with gestational age from 32 to 36 weeks (50.5%). These and other characteristics of the assessed group are demonstrated in Table 1.
BPD was observed in 63 (21.5%) newborns. Table 2 presents the results of bivariate analyses regarding associations between pregnancy and birth conditions and characteristics of preterm newborns and BPD occurrence. The variables with most remarkable descriptive levels or statistical significance were birth weight and gestational age. Table 3 presents the results of bivariate analyses regarding the associations between intercurrences and morbidities identified for neonates during NICU stay and presence of BPD. In this table, almost every assessed intercurrences presented a remarkable descriptive level or statistical significance (with
p<0.001).
After multiple analysis, the variables that remained statistically associated with BPD were: gestational age, birth weight and registration of late onset sepsis, according to Table 4.
DiscussionThis study revealed a high incidence of BPD for the group assessed, particularly considering that it approached only children followed-up in an outpatient clinic for high-risk newborns. In other words, it is a value registered only among survivors. The literature registers diverging values for BPD occurrence, since there is no standardization of groups assessed. In Brazil and Latin America, the values vary from 20.0% to 30.0%,
7,12-15 according to the group of neonates or location assessed.
A survey carried out in Cuba including only newborns of extreme low birth weight identified a incidence of 20%.
15 In Argentina, also evaluating neonates with birth weight under 1500 grams, the observed incidence was 22%.
7 Other countries registered lower values, such as the neonatal networks of Canada and Japan, with rates that varied from 12.3% to 14.6%, respectively, for extreme low weight newborns.
16 However, there are registries of a higher incidence, such as 28.5% in Spain
17 or 26.2% to 30.4% in Vermont Oxford Network, in the United States.
18Nevertheless, it is relevant to highlight that some of these surveys, in spite of being accurate, have more than ten years of publication.
High BPD values, as observed in this study, highlight the need for review of assistance practices of health teams for both antenatal and postnatal care. The literature indicates that BPD predictors are multifactorial and may have association with prenatal, perinatal and postnatal conditions.
3,4,6-8,19 In this study, variables that were associated with BPD occurrence were birth weight, gestational age and late-onset sepsis.
Actually, preterm birth and low birth weight stand out as the main and strongest factors associated with BPD, with registries of incidence and severity inversely proportional for both gestational age and birth weight.
4,7,14,19 There is a structural and functional immaturity in several systems related to preterm neonate and/or low weight neonate due to preterm birth that determinate the need for interventions aiming to preserve life. Within these measures, we highlight the need for supplemental oxygen, mostly with mechanical ventilation support. These factors, isolated or jointly, are intrinsically associated with oxidative stress and hyperoxia, which imply in direct damage to the preterm lung, interrupting the process of lung development and maturation.
19 Surveys carried out with animal models demonstrated that the exposition to high concentrations of oxygen themselves can induce inflammation, fibrosis and emphysema in immature lungs.
20 Under the physiopathological perspective, hyperoxia results in lesions and death of endothelial cells, followed by epithelial cell damage and rupture of alveolar-capillary membrane, with subsequent impairment of gas exchange.
19It is relevant to observe that birth weight under 1000 grams was not an associated variable in the final model, although the confidence interval indicated a non-negligible trend. In the author’s perspective, this fact may occur due to the fact of the study only approached surviving neonates. In this regard, extreme low weight neonates who had BPD were not included in the study since they did not survive due to this condition or other intercurrences. Although there is a very close correlation between birth weight and gestational age, these variables indicate distinct characteristics regarding the mechanisms of BPD development. In a study carried out with preterm twins, it was observed that the divergent intrauterine growth was a determinant factor in the morbidity and mortality outcomes. Among the slightly divergent pairs, it was observed a higher risk of BPD or death. For the highly divergent pairs, the lower one presented higher incidence of sepsis, use and permanence of mechanical ventilation and BPD, besides higher mortality.
21Although several factors registered in the literature as postnatal predictors of BPD have been identified in the bivariate analyses of this study, only late-onset sepsis stood in the final model. This association was already indicated by other authors.
15,17,22-25 Some authors reasoned that, among post-neonatal factors associated with BPD, there is common mechanism characterized by the production of inflammatory cytokines, which may occur by means of the respiratory distress syndrome itself, of the invasive mechanical ventilation, the oxygen supply and sepsis, and the latter leads to both local and systemic inflammatory processes, simultaneously.
22,23 In a prospective cohort study carried out with data collected from 64 Korean NICUs, it was observed that BPD incidence was significantly associated with late-onset sepsis. The study also observed an increase for the risk of repeated cases of late-onset sepsis and higher severity in cases of fungal sepsis.
22Besides the physiopathological mechanisms directly involved with the infectious process, late-onset sepsis is, frequently, a severe complication, usually followed by hemodynamic alterations and diverse clinical manifestations, and the germs involved are often of nosocomial origin, including fungi.
26 Due to the severity associated with late-onset sepsis, most neonates affected need to start or maintain mechanical ventilation support, and, in this perspective, supplemental oxygen and positive mechanical ventilation are themselves risk factors for BPD.
2,4,19There are authors who identified other factors associated with BPD that were not identified in this study, such as, for example, the use of surfactant,
14 the need for resuscitation in birth room,
15 patent ductus arteriosus
7,13 and mechanical ventilation.
7,13,14,17 Although these factors have been identified in the early analysis (bivariate), they did not persisted in the final model. This fact may occur due to the particularities of the groups assessed in each study. Beyond the results observed in this study, it is relevant that professionals are aware of the implementation of strategies and assistance practices that should be performed in order to mitigate BPD occurrence. The impacts and synergic effects of perinatal interventions based on evidence about primary outcomes of BPD have been recently described in the literature and include the use of antenatal corticosteroid, birth in facilities with tertiary NICUs, prevention of intubation in birth rooms, early caffeine therapy and early extubation.
27With regard to antenatal corticosteroids, a cross-sectional study that assessed over 11,000 neonates concludedthat for those between 22 and 28 weeks of gestational age, in which the exposition to corticosteroids, compared to the absence of exposition, is associated with lower mortality rates, however the BPD rate did not differ.
28 On the other hand, two systematic reviews from the Cochrane Organization, one of them with 30 surveys and the other, 27 studies, demonstrated a significant impact on the decrease of BPD rates, comparing the use of corticosteroids with placebo or no intervention.
29,30 In other words, the most recent evidence of high quality suggests that the use of prenatal corticosteroids is effective for the reduction of BPD risk in preterm newborns and supports the use of prenatal corticosteroids as an important intervention to improve respiratory outcomes in preterm neonates.
The results observed should be considered concerning some limitations. In this study, given the above, the group assessed was identified within surviving neonates that were followed-up in an outpatient clinic. Thus, it is possible that other variables were associated from a concurrent cohort. It was not possible to classify the severity of BPD, and in this perspective, it is not possible to establish accurate alert signs for professionals of the services involved. In spite of these considerations, the study has important results for the region, indicating the need for interventions in mother-and-child care, both in prenatal care, in order to reduce prematurity and low birth weight, and postnatal care, for the reduction of infectious cases. In conclusion, we observed a high incidence of bronchopulmonary dysplasia for the group assessed, which was associated with prematurity, low birth weight and late-onset sepsis. These factors draw attention to the need for more effective policies that improve prenatal assistance and care in Neonatal Intensive Care Units.
References1. Ohuma EO, Moller AB, Bradley E, Chakwera S, Hussain-Alkhateeb L, Lewin A,
et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet. 2023; 402 (10409): 1261-71.
2. Bedi PK, DeHaan K, MacLean JE, Castro-Codesal ML. Predictors of longitudinal outcomes for children using long-term noninvasive ventilation. Pediatr Pulmonol. 2021; 56 (5): 1173-81.
3. Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M,
et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am J Respir Crit Care Med. 2019; 200 (6): 751-9.
4. Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV,
et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J Pediatr. 2018; 197: 300-8.
5.. Siffel C, Kistler KD, Lewis JFM, Sarda SP. Global incidence of bronchopulmonary dysplasia among extremely preterm infants: a systematic literature review. J Matern Fetal Neonatal Med. 2021; 34 (11): 1721-31.
6. Yang Y, Li J, Mao J. Early diagnostic value of C-reactive protein as an inflammatory marker for moderate-to-severe bronchopulmonary dysplasia in premature infants with birth weight less than 1500 g. Int Immunopharmacol. 2022; 103: 108462.
7. Brener Dik PH, Niño Gualdron YM, Galletti MF, Cribioli CM, Mariani GL. Bronchopulmonary dysplasia: incidence and risk factors. Arch Argent Pediatr. 2017; 115 (5): 476-82.
8. Hwang JS, Rehan VK. Recent Advances in Bronchopulmonary Dysplasia: Pathophysiology, Prevention, and Treatment. Lung. 2018; 196 (2): 129-38.
9. Ibrahim J, Bhandari V. The definition of bronchopulmonary dysplasia: an evolving dilemma. Pediatr Res. 2018; 84: 586-8.
10. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001; 163: 1723-9.
11. Instituto Brasileiro de Geografia e Estatística (IBGE). Cidades e Estados. 2024. [acesso em 2024 Jan 15]. Disponível em:
https://www.ibge.gov.br/cidades-e-estados/mg/montes-claros.html12. Tapia JL, Agost D, Alegria A, Standen J, Escobar M, Grandi C,
et al.; NEOCOSUR Collaborative Group. Bronchopulmonary dysplasia: incidence, risk factors and resource utilization in a population of South American very low birth weight infants. J Pediatr. 2006; 82: 15-20.
13. Freitas BAC, Peloso M, Silveira GL, Longo GZ. Prevalência e fatores associados à displasia broncopulmonar em hospital de referência para microrregião de Minas Gerais. Rev Bras Ter Intensiva. 2012; 24 (2): 179-83.
14. Carillo-Franco J, Guevara-Suta S, Mendoza-Romero D. Displasia broncopulmonar y surelación com los cuidados respiratorios en prematuros menores de 32 semanas en una unidad neonatal, Bogotá 2017. Medicas UIS. 2021; 34 (2): 41-7.
15. Zavaleta-Gutierrez FE, Concepción-Urteaga LA, Concepción-Zavaleta MJ, Aguilar-Villanueva DA. Factores de riesgo y displasia broncopulmonar em reciénnacidos prematuros de muy bajo peso al nacer. Rev Cuba Pediatr. 2019; 91 (1): e600.
16. Isayama T, Lee SK, Mori R, Kusuda S, Fujimura M, Ye XY,
et al.; Canadian Neonatal Network; Neonatal Research Network of Japan. Comparison of mortality and morbidity of very low birth weight infants between Canada and Japan. Pediatrics. 2012; 130 (4): e957-65.
17. Alonso AS, Díaz SP, Soto RS, Ávila-Álvarez A. Epidemiología y factores de riesgo asociados a displasia broncopulmonar en prematuros menores de 32 semanas de edad gestacional. An Pediatr. 2022; 96 (2): 242-51.
18. Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012; 129 (6): 1019-26.
19. Thekkeveedu RK, Guaman MC, Shivanna B. Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Respir Med. 2017; 132: 170-7.
20. Ambalavanan N, Morty RE. Searching for better animal models of BPD: A perspective. Am J Physiol Lung Cell Mol Physiol. 2016; 311 (5): L924-7.
21. Sabatelli D, Milet B, Mena P, Domínguez A. Growth restriction increases the risk of bronchopulmonary dysplasia, death, and sepsis in twins of 30 weeks or less of gestation. Rev Chil Pediatr. 2019; 90 (1): 36-43.
22. Jung E, Lee BS. Late-onset sepsis as a risk factor for bronchopulmonary dysplasia in extremely low birth weight infants: a nationwide cohort study. Sci Rep. 2019; 9: 15448.
23. Lahra MM, Beeby PJ, Jefery HE. Intrauterine infammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics. 2009; 123: 1314-9.
24. Shah J, Jefferies AL, Yoon EW, Lee SK, Shah PS; Canadian Neonatal Network. Risk Factors and Outcomes of Late-Onset Bacterial Sepsis in Preterm Neonates Born at<32 Weeks’ Gestation. Am J Perinatol. 2015; 32 (7): 675-82.
25. Ebrahimi ME, Romijn M, Vliegenthart RJS, Visser DH, van Kaam AH, Onland W. The association between clinical and biochemical characteristics of late-onset sepsis and bronchopulmonary dysplasia in preterm infants. Eur J Pediatr. 2021; 180 (7): 2147-54.
26. Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017; 390 (10104): 1770-80.
27. Chen X, Yuan L, Jiang S, Gu X, Lei X, Hu L,
et al. Synergistic effects of achieving perinatal interventions on bronchopulmonary dysplasia in preterm infants. Eur J Pediatr. 2024; 183: 1711-21.
28. Travers CP, Carlo WA, McDonald SA, Das A, Bell EF, Ambalavanan N,
et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. Am J Obstet Gynecol. 2018 Jan; 218 (1): 130.e1-130.e13.
29. Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017 Mar; 3 (3): CD004454.
30. McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020 Dec; 12 (12): CD004454.
Author’s contributionMenezes MSD: idealization and coordination of the research project, data collection and analysis, writing and review of the manuscript. Dias VF: data collection and analysis, writing and review of the manuscript. Araújo DD, Carneiro JA and Pinho L: data analysis, writing and review of the manuscript. Caldeira AP: idealization and coordination of the research project, data analysis, writing and review of the manuscript. All authors approve the final version of the article and declare no conflict of interest.
Received on August 29, 2023
Final version presented on July 3, 2024
Approved on July 18, 2024
Associated Editor: Karla Bomfim
 ; Victor Figueiredo Dias 2
; Victor Figueiredo Dias 2 ; Diego Dias de Araújo 3
; Diego Dias de Araújo 3 ; Jair Almeida Carneiro 4
; Jair Almeida Carneiro 4 ; Lucineia de Pinho 5
; Lucineia de Pinho 5 ; Antônio Prates Caldeira 6
; Antônio Prates Caldeira 6