ABSTRACT
OBJETIVOS: avaliar a associação entre o tempo para iniciar o primeiro contato pele a pele (CPP) e o tempo diário praticado com a taxa de sepse tardia em recém-nascidos ≤1.800g.
MÉTODOS: coorte multicêntrica realizada em unidades neonatais de três regiões geográficas brasileiras. O CPP foi registrado em ficha individual pela equipe e pais do recém-nascido. Dados maternos e neonatais foram obtidos por questionários aplicados às mães e em prontuários médicos. A análise dos dados foi realizada por algoritmo da árvore de classificação, que dividiu o conjunto de dados em subconjuntos mutuamente exclusivos que melhor descreveram a variável resposta.
RESULTADOS: 405 recém-nascidos participaram do estudo, com média de 31,3±2,7 semanas de idade gestacional e mediana de peso ao nascer 1.412g (IQ=1.164-1.605g). Realizar o primeiro CPP com até 137h de vida (≤5,7 dias) foi associado a menor taxa de sepse tardia (p=0,02) para recém-nascidos que fizeram CPP diário de 112,5 a 174,7 min/dia (1,9 a 2,9h/dia), com redução na taxa de sepse (39,3% para 27,5%). Além disso, a duração do CPP>174,7min/dia (>2,9h/dia) foi relevante (p<0,001) para os recém-nascidos >1.344g, com redução nesse desfecho (21,1% para 6%).
CONCLUSÕES: o CPP mostrou-se importante para redução das taxas de sepse tardia em recém-nascidos pré-termo, especialmente quando realizado de forma oportuna (≤5,7 dias) e prolongada (>2,9h/dia).
Keywords:
Método canguru, Recém-nascido de baixo peso, Recém-nascido pré-termo, Sepse neonatal
RESUMO
OBJETIVOS: avaliar a associação entre o tempo para iniciar o primeiro contato pele a pele (CPP) e o tempo diário praticado com a taxa de sepse tardia em recém-nascidos ≤1.800g.
MÉTODOS: coorte multicêntrica realizada em unidades neonatais de três regiões geográficas brasileiras. O CPP foi registrado em ficha individual pela equipe e pais do recém-nascido. Dados maternos e neonatais foram obtidos por questionários aplicados às mães e em prontuários médicos. A análise dos dados foi realizada por algoritmo da árvore de classificação, que dividiu o conjunto de dados em subconjuntos mutuamente exclusivos que melhor descreveram a variável resposta.
RESULTADOS: 405 recém-nascidos participaram do estudo, com média de 31,3±2,7 semanas de idade gestacional e mediana de peso ao nascer 1.412g (IQ=1.164–1.605g). Realizar o primeiro CPP com até 137h de vida (≤5,7 dias) foi associado a menor taxa de sepse tardia (p=0,02) para recém-nascidos que fizeram CPP diário de 112,5 a 174,7 min/dia (1,9 a 2,9h/dia), com redução na taxa de sepse (39,3% para 27,5%). Além disso, a duração do CPP>174,7min/dia (>2,9h/dia) foi relevante (p<0,001) para os recém-nascidos >1.344g, com redução nesse desfecho (21,1% para 6%).
CONCLUSÕES: o CPP mostrou-se importante para redução das taxas de sepse tardia em recém-nascidos pré-termo, especialmente quando realizado de forma oportuna (≤5,7 dias) e prolongada (>2,9h/dia).
Palavras-chave:
Método canguru, Recém-nascido de baixo peso, Recém-nascido pré-termo, Sepse neonatal
IntroductionNeonatal deaths account for 44% of infant mortality in developing countries and are mainly related to prematurity, asphyxia at birth and the occurrence of infections in newborns (NB).
1 In order to reduce unfavorable neonatal outcomes, such as reducing late-onset neonatal sepsis, the World Health Organization (WHO) has recommended the practice of skin-to-skin contact (SSC), which should be started as soon as the NB has reached clinical stability.
2SSC is a light technology that consists of placing the NB, free of clothing, in skin-to-skin contact on the mother's or father's chest, in a prone position, for as long as it is comfortable for both. Initially conceptualized in Colombia in 1978, it has spread to several countries around the world and has been the subject of important studies that have proven its effectiveness in reducing morbidity and mortality in the neonatal period.
3,4Studies have pointed out to the influence of different times when SSC is practiced in reducing late-onset sepsis rates. In an investigation carried out in France, the authors observed an association between SSC performed for three hours or more a day with a reduction in late-onset sepsis;
5 the same association was observed in a study in Ukraine,
6 while in the Philippines this association occurred with SSC time greater than or equal to four hours/day.
7 Another multicenter, randomized study that analyzed immediate SSC, observed a reduction in rates of suspected sepsis with a median time on the first contact of 1.3 hours and a daily practice of SSC in the neonatal intensive care unit (NICU) of 16.9 hours.
8Meta-analyses carried out in important systematic reviews did not find conclusive results regarding the daily time of SSC associated with a lower infection rate in NB low birth weight due to the great variation in the time used in each primary study.
3,4 Therefore, there is currently no consensus on the appropriate time to start this first contact after the NB is admitted to Neonatal Units, as well as on what daily time for SSC is necessary to observe an effect in reducing late sepsis rates.
The aim of this study was to assess the association between the time to initiate the first SSC and the daily time practiced with the rate of late-onset sepsis in newborns weighing ≤1,800g during neonatal hospitalization.
MethodsThis is a multicenter observational prospective cohort study carried out between May 2018 and March 2020. Five Brazilian neonatal units participated in this study, two were from the Northeast, two from the Southeast and one from the South.
All live births in these institutions during the study period that met the following criteria were considered eligible: single childbirth, birth weight up to 1,800g, no malformations, severe perinatal asphyxia and/or genetic syndromes. The non-inclusion and exclusion criteria, as well as the losses, are detailed in the sample flowchart (Figure 1). Women with psychiatric or behavioral disorders and HIV-positive (Human Immunodeficiency Virus) were not included in the study. Some women were excluded from the sample according to the criteria detailed in Figure 1.
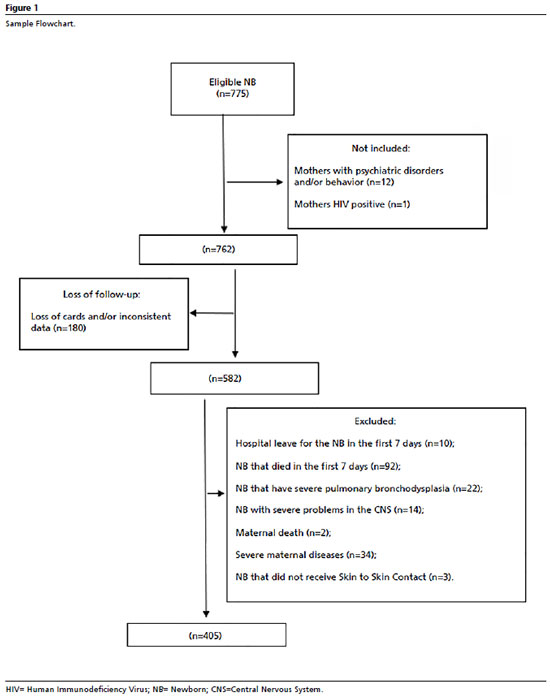
For this reseach, the minimum sample size was estimated at 357 dyads. This quantitative was based on a pilot study which considered an exposed/unexposed ratio of 06 and a risk difference of 23%. A bilateral significance level of 95%, power of 99% and type I error of 5% were considered. The pilot study was carried out in the first stage of the multicenter project, implemented in May 2018, with the aim of establishing parameters that would enable sample calculation, as well as providing data that was incorporated into the total study sample. The calculation of 357 dyads refers to the minimum necessary to represent the reliability of the results, however the total sample exceeded the number of participants to 405 dyads.
Maternal and neonatal variables were collected using questionnaires administered to mothers and supplemented with data from medical records during the neonate's hospitalization. The length of SSC was registered on cards attached to the hospital bed and filled in by the health team after hospitalization to the neonatal unit. Parents were also trained to fill in the SSC registration, under the supervision of the health team. The card registration has a start and end time for each SSC, as well as who performed it (mother or father). These registrations were checked daily by previously trained auxiliary researchers who made contact with the parents and health professionals, as well as consolidating the data in the NB's medical records.
For the statistical analysis was considered, the outcome variable "late-onset sepsis", was defined when its diagnostical evidence (clinical/laboratory/microbiological) occurred after the first 48 hours of the newborn's life, while they were hospitalized in a neonatal care unit, duly registered in the medical records.
9The explanatory variables were defined from a theoretical model, based on findings in the literature, to investigate the association with the outcome under study.
The explanatory variables for women were: age (under 20, 20 to 34 and over 35); schooling (no schooling/incomplete primary schooling; complete primary schooling/incomplete secondary schooling; complete secondary schooling/incomplete higher education and complete higher education); marital status (with partner and without partner); prenatal adequacy (adequate or no prenatal care / inadequate), prenatal care was considered adequate if it began up to the 4
th month of pregnancy and six or more consultations were made for a full-term pregnancy or fewer according to gestational age at the time of delivery (three consultations up to 29 weeks; four consultations between 30 and 33 weeks and five consultations between 34 and 36 weeks),
10 pregnancy-specific hypertensive syndrome (PSHS); history of infection during pregnancy; use of alcohol during pregnancy; use of corticosteroids before delivery and birth route (vaginal or cesarean).
The newborn's variables were: birth weight, measured in grams; gestational age at birth, calculated by the date of the last menstruation or by first trimester ultrasound or by the New Ballard score, expressed in days and transformed into weeks for analysis; weight adequacy for gestational age, categorized as AIG, PIG or GIG (adequate, small or large for gestational age), according to the Intergrowth 21 second classification;
11 Score for Neonatal Acute Physiology - Perinatal Extension II (Snappe II), scored from 0 to 162;
12 APGAR at the 5
th minute of life, scored from 0 to 10;
13 early infection, when it occurred within the first 48 hours of the newborn's life.
9The variable referring to the daily time of exposure to SSC during hospitalization days was measured in total minutes, then calculated in minutes per day (total time of SSC during hospitalization divided by the number of days in which this contact was made with one of the parents).
The variable referring to the first SSC was registered as the total number of hours in which this first contact took place after the NB's hospitalization.
The data collected was tabulated in a Google Form tool and then exported to a Microsoft Office Excel spreadsheet, version 2016. Quantitative data was represented by mean and standard deviation or medians and interquartile ranges, depending on its distribution and normality criteria, and categorical variables were presented as frequencies and percentages.
For the statistical analysis of the theoretical model with the selected variables identified above, a non-parametric method was used, based on learning machine (artificial intelligence): the Classification Tree.
14 The dependent variable used was "late-onset sepsis", considered as the "root node" of this tree. Next, the variable inserted was "SSC/day" was inserted as the first "descendant node", and from there, using logical tests, an algorithm was generated which was selected and identified as the "other descendant nodes" from the set of maternal and child predictor variables defined in the theoretical model.This process was carried out until there was no further division of these nodes, ending the growth of the tree into "terminal nodes". By analyzing continuous variables, the algorithm determined the most statistically appropriate cut-off points.
15 Thus, the aim of this process was to find the cut-off points for SSC time that were associated with late-onset sepsis during hospitalization.To build the classification tree, the entire set of selected maternal and child variables was entered into IBM SPSS 21 software.
The descriptive results of the sample were analyzed using the Stata 14.0 statistical package. For data analysis, significance levels were set at 5% and 95% confidence intervals (CI95%) were adopted.
The research was approved by the institutional Research Ethics Committee, under opinion number 2.570.959 (CAAE: 83803817.0.1001.5086).
ResultsA total of 405 dyads were analyzed (Figure 1), with a mean gestational age of 31.3 ± 2.7 weeks and a median weight of 1,412 (1,164-1,605) grams. The rate of late-onset sepsis during hospitalization was 29.1%. Other newborns' characteristics are shown in Table 1and Table 2 describing the maternal characteristics.
Figure 2 presents the classification tree with the outcome of late-onset sepsis, according to maternal and newborn characteristics. This tree has eight nodes, five of which are terminal. The tree shows the relationship between the dyads characteristics at three levels of depth, with none of the maternal characteristics remaining associated with the outcome in the explanatory model. The most relevant characteristics found were: onset time to the first SSC ≤137h (5.7 days) and NB's birth weight >1,344 grams.
The introduction of SSC time/day as the explanatory variable closest to the outcome generated three descending nodes (SSC ≤112.5; between 112.5-174.7 and >174.7 min/day), but showed no significant association with this variable (
p=0.42). However, the second node showed that the NB group who practiced SSC daily was between 112.5 and 174.7 min/day (1.9 hours/day to 2.9 hours/day), when they performed their first SSC up to 137 hours old (≤5.7 days) was associated with lower rates of late sepsis than those who did it later (27.5 versus 54.7%), which represented a reduction in late sepsis rates from 39.3% to 27.5% in this group.
The third node showed that NBs who performed SSC daily was >174.7 min/day (>2.9 hours/day) and were born weighing >1,344 grams had lower rates of late-onset sepsis (6%) compared to those who were born weighing less (53.2%), which represented a reduction of 24.1% to 6% in the rates of late-onset sepsis in this group.
DiscussionThis study showed that NB who underwent SSC between 112.5 and 174.7 minutes/day (1.9 hours to 2.9 hours/day) and had their first contact after birth within 137 minutes of life (≤5.7 days) had lower rates of late-onset sepsis, with a reduction in these rates from 39.3% to 27.5%. It was also observed that performing SSC daily for longer than 174.7 min/day (>2.9 hours/day) is associated with lower rates of late-onset sepsis for NB born weighing >1,344g, with a reduction in late-onset sepsis from 24.1% to 6% in this group.
Important systematic reviews have shown that newborns with low birth weight (<2,500 grams) who underwent SSC during hospitalization had a lower rate of late-onset sepsis compared to those who underwent conventional care.
3,4 The present study showed that performing the first SSC in a more timely manner (≤5.7 days of the newborn's life) significantly modifies the rates of late-onset sepsis in preterm newborns. Arya
et al.
16 observed that mortality related to neonatal sepsis was 37% lower in the NB group who underwent SSC within the first two hours of life. Other studies have pointed to the importance of early initiation of this contact, after hospitalization to the NICU, as a form of protection against these infections.
5,6,8 Current findings also point out to the initiation time of the first SSC as an important factor in reducing rates of late sepsis, indicating that the opportune time for this first contact should not be missed.
One theory that could explain the protection of SSC performed earlier in reducing late-onset sepsis concerns the protection of the microbiome of the NB admitted to the NICU. The human microbiome influences the immune system and plays a significant role in both the prevention and acquisition of the disease issues. Preterm neonates are more vulnerable in developing an altered microbiome due to the direct and prolonged contact that they are exposed to in an atypical environment such as the NICU.
17 Another hypothesis for altering the microbiome would be the form of birth.
Children born through the vaginal have intestinal microbial content similar to that of the maternal vaginal and intestinal flora, which includes commensal bacteria such as
Bacteroides, Bifidobacterium, Clostridium and lactic acid bacteria, while those born by cesarean section are colonized by skin bacteria (such as
Staphylococcus,
Corynebacterium,
Propionibacterium sp.) which delays the colonization and establishment of those commensal bacteria in the NB's intestine.
18,19 In addition, preterm NBs have low bacterial diversity, composed of a higher proportion of
Proteobacteria and
Enterococcus in the gut compared to non-preterm NBs.
20Due to these factors, preterm NBs, usually born by cesarean section, with low diversity in the intestinal microbiome and exposed to a complex hospital environment such as the NICU, whose microbiological flora is often formed with pathogenic strains, are more prone in developing late-onset neonatal sepsis than NBs at term.
17 A Brazilian study has shown that it is possible to decolonize hospital bacterial strains through maternal commensal flora exchanges, associated with daily SSC.
21 Therefore, the earlier and more regularly SSC is carried out, the greater the possibility of the non-pathogenic flora of the mother and father colonizing the NB's body, which could have a significant impact on reducing late-onset sepsis rates.
Birth weight (>1,344g) was also another variable associated with a lower rate of late-onset sepsis in the current study, especially for the NB group who underwent SSC daily for a longer time (>2.9 hours/day). According to the Intergrowth scale 21, NB <1,344g correspond, in large proportion, to the very preterm,
11 and this group is subject to additional complications such as intraventricular hemorrhage, retinopathy and necrotizing enterocolitis.
22 In addition, they remain in the hospital for long periods, which contributes to greater exposure to hospital pathogens in a body that is more fragile due to its biological immaturity, justifying a high infection rate found in this group. However, SSC should be encouraged in this group of very preterm infants, in order to contribute to other benefits already demonstrated in the literature, such as maternal bonding,
23 shorter time to reach a complete enteral diet,
24 exclusive breastfeeding,
25,26 neuroprotection,
27 stress reduction,
28 among others.
On the other hand, in the present study, preterm NBs with a birth weight greater than 1,344g benefited from longer use of SSC (>2.9 hours/day) in reducing rates of late-onset sepsis. This demonstrates the impact that longer practice of this technology can have on the control of neonatal infections.
A WHO clinical trial showed a reduction in neonatal deaths and cases of suspected sepsis in the group that performed the first SSC immediately (median 1.3 hours) and for a longer daily time during NICU stay (median 16.9 hours/day) compared to the control group.
8 Another clinical trial, carried out in the Philippines, found that practicing SSC for at least four hours a day was associated with a reduction in morbidities (necrotizing enterecolitis and neonatal sepsis) in preterm infants undergoing nasal Continuous Positive Airway Pressure (CPAP) respiratory support.
7 A systematic review with meta-analysis showed greater benefits on neonatal mortality and a probable reduction in neonatal clinical sepsis when SSC was performed for at least eight hours a day.
29Some authors theorize that the reduction in infection and mortality rates is due to the possibility of the NB being colonized with the maternal microbiome, besides a greater likelihood of early breastfeeding, less handling of the NB by other people, a reduction in stress levels due to the mother's presence and the mother's constant monitoring of her child.
8,20,28 Therefore, the mother's presence in the NICU, practicing SSC for a longer period of time, is of fundamental importance in order to achieve lower rates of late-onset sepsis and, consequently, a reduction in neonatal deaths.
A limitation of this study is that the neonatal units of the different study centers were not evaluated as independent variables, which may have influenced the analysis of late-onset sepsis rates.
The participation of five institutions from three different regions of the country can be considered a positive point of the study, as well as the fact that the data was collected prospectively. Also noteworthy was the choice of a method that allowed us to observe cut-off points for the time of the onset and daily contact of SSC associated with late infections.Using the "Classification Tree",
14 we were able to create an explanatory model for this outcome, which was adjusted by various variables, and place the practice of SSC in this context.
This study identified a time of up to 137 minutes (≤ 5.7 days) to perform the first SSC in the NICU and observed a positive effect on reducing late sepsis in neonates who practiced 112.5 to 174.7 minutes/day (1.9 hours to 2.9 hours/day) of SSC. It also showed that longer SSC during neonatal hospitalization (>2.9 hours/day) was associated with a significant reduction in late-onset sepsis in those born weighing more than 1,344g. Studies that present cut-off points for the start time and duration of daily SSC practice are important to guide health teams to work in neonatal care.
References1. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J,
et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016 Dec; 388 (10063): 3027-35.
2. World Health Organization (WHO). WHO recommendations on interventions to improve preterm birth outcomes. Geneva: WHO; 2015. [access in 2020 Nov 10]. Available from:
https://www.who.int/publications/i/item/97892415089883. Conde-Agudelo A, Díaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2016 Aug; 2016 (8): CD002771.
4. Boundy EO, Dastjerdi R, Spiegelman D, Fawzi WW, Missmer SA, Lieberman E,
et al. Kangaroo mother care and neonatal outcomes: a meta-analysis. J Paediatr Child Health. 2016; 52 (5): 579.
5. Casper C, Sarapuk I, Pavlyshyn H. Regular and prolonged skin-to-skin contact improves short-term outcomes for very preterm infants: A dose-dependent intervention. Arch Pédiatr. 2018 Nov; 25 (8): 469-75.
6. Pavlyshyn H, Sarapuk I, Casper C, Makieieva N. Kangaroo mother care can improve the short-term outcomes of very preterm infants. J Neonatal Perinatal Med. 2021 Jun; 14 (1): 21-8.
7. Ricero-Luistro CP, Villanueva-Uy MET, Libadia AGI, De Leon-Mendoza S. Effectiveness of kangaroo mother care in reducing morbidity and mortality among preterm neonates on nasal continuous positive airway pressure: a randomized controlled trial. Acta Medica Philippina. 2021 Dec; 55 (9).
8. WHO Immediate KMC Study Group, Arya S, Naburi H, Kawaza K, Newton S, Anyabolu CH,
et al. Immediate "Kangaroo Mother Care" and Survival of Infants with Low Birth Weight. New Engl J Med. 2021 May; 384 (21): 2028-38.
9. Agência Nacional de Vigilância Sanitária (ANVISA). Critérios Diagnósticos de Infecção Associada à Assistência à Saúde – Neonatologia 3. Brasília (DF): Anvisa; 2017. [access in 2020 Nov 10]. Available from:
https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/servicosdesaude/publicacoes/caderno-3-criterios-diagnosticos-de-infeccao-associada-a-assistencia-a-saude-neonatologia.pdf10. Goudard MJF, Simões VMF, Batista RFL, Queiroz RCS, Alves MTSSB, Coimbra LC,
et al. Inadequacy of prenatal care content and associated factors in a cohort in northeastern Brazil. Ciênc Saúde Colet. 2016; 21 (4): 1227-38.
11. Oxford University. Intergrowth-21st. Oxford, 2020. [
Internet]. [access in 2020 Aug 10]. Available from:
http://intergrowth21.ndog.ox.ac.uk/pt12. Muktan D, Singh RR, Bhatta NK, Shah D. Neonatal mortality risk assessment using SNAPPE- II score in a neonatal intensive care unit. BMC Pediatr. 2019 Aug; 19 (1): 279.
13. Simon LV, Hashmi MF, Bragg BN. Score APGAR. In: StatPearls. StatPearls Publishing, Treasure Island (FL); 2022. PMID: 29262097
14. Bertsimas D, Dunn J. Optimal classification trees. Machine Learning. 2017 Apr; 106 (7): 1039-2.
15. Abdar M, Zomorodi-Moghadam M, Das R, Ting I-Hsien. Performance analysis of classification algorithms on early detection of liver disease. Expert Syst Appl. 2017 Jan; 67: 239-51.
16. Arya S, Chhabra S, Singhal R, Kumari A, Wadhwa N, Anand P,
et al. Effect on neonatal sepsis following immediate kangaroo mother care in a newborn intensive care unit: a post-hoc analysis of a multicentre, open-label, randomised controlled trial. EClinical Medicine. 2023 May; 60: 102006.
17. Hartz LE, Bradshaw W, Brandon DH. Potential NICU Environmental Influences on the Neonateʼs Microbiome. Adv Neonatal Care. 2015 Oct; 15 (5): 324-35.
18. Faa G, Gerosa C, Fanni D, Nemolato S, van Eyken P, Fanos V. Factors influencing the development of a personal tailored microbiota in the neonate, with particular emphasis on antibiotic therapy. J Matern Fetal Neonatal Med. 2013 Sep; 26 (Supl. 2): 35-43.
19. Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015 Feb; 21 (2): 109-17.
20. Dahl C, Stigum H, Valeur J, Iszatt N, Lenters V, Peddada S,
et al. Preterm infants have distinct microbiomes not explained by mode of delivery, breastfeeding duration or antibiotic exposure. Int J Epidemiol. 2018 Oct; 47 (5): 1658-69.
21. Lamy Filho F, Sousa SHC, Freitas IJS, Lamy ZC, Simões VMF, Silva AAM,
et al. Effect of maternal skin-to-skin contact on decolonization of Methicillin-Oxacillin-Resistant Staphylococcus in neonatal intensive care units: a randomized controlled trial. BMC Pregnancy Childbirth. 2015; 15 (1): 1-7.
22. Bonamy AKE, Zeitlin J, Piedvache A, Maier RF, Heijst A, Varendi H,
et al. Wide variation in severe neonatal morbidity among very preterm infants in European regions. Arch Dis Child Fetal Neonatal Ed. 2019; 104 (1): F36-45.
23. Caetano C, Pereira BB, Konstantyner T. Effect on the practice of the kangaroo method on the formation and strengthening of the mother-baby bond: a systematic review. Rev Bras Saúde Matern Infantil. 2022; 22 (1): 11-22.
24. Pandya D, Kartikeswar GAP, Patwardhan G, Kadam S, Pandit A, Patole S. Effect of early kangaroo mother care on time to full feeds in preterm infants: a prospective cohort study. Early Hum Dev. 2021; 154: 105312.
25. Jayaraman D, Mukhopadhyay K, Bhalla AK, Dhaliwal LK. Randomized Controlled Trial on Effect of Intermittent Early Versus Late Kangaroo Mother Care on Human Milk Feeding in Low-Birth-Weight Neonates. J Human Lactation. 2017 Feb; 33 (3): 533-9.
26. Goudard MJF, Lamy ZC, Marba STM, Lima GMS, Santos AM, Vale MS,
et al. The role of skin-to-skin contact in exclusive breastfeeding: a cohort study. Rev Saúde Pública 2022; 56 (71): 1-10.
27. Hardin JS, Jones NA, Mize KD, Platt M. Parent-Training with Kangaroo Care Impacts Infant Neurophysiological Development & Mother-Infant Neuroendocrine Activity. Infant Behav Dev. 2020 Feb; 58: 101416.
28. Ionio C, Ciuffo G, Landoni M. Parent-Infant Skin-to-Skin Contact and Stress Regulation: a systematic review of the literature. Int J Envir Res Public Health. 2021 Apr 28; 18 (9): 4695.
29. Sivanandan S, Sankar MJ. Kangaroo mother care for preterm or low birth weight infants: a systematic review and metaanalysis. BMJ Global Health. 2023; 8: e010728.
Acknowledgements: We would like to thank the
Fundação de Amparo à Pesquisa e Desenvolvimento Tecnológico do Maranhão (FAPEMA) and the
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), for funding this research.
Authors’ contribution: Lamy Filho F, Goudard MJF, Marba STM, Santos AM, Lima GMS, Costa R, Azevedo VMGO and Lamy ZC participated in the conception of the project, data collection, data analysis and writing of the manuscript.
All the authors have approved the final version of the article and declare no conflicts of interest.
Received on June 5, 2023
Final version presented on March 25, 2024
Approved on March 26, 2024
Associated Editor: Priscila Mullachery
 ; Marivanda Julia Furtado Goudard2
; Marivanda Julia Furtado Goudard2 ; Sérgio Tadeu Martins Marba3
; Sérgio Tadeu Martins Marba3 ; Alcione Miranda dos Santos4
; Alcione Miranda dos Santos4 ; Geisy Maria de Souza Lima5
; Geisy Maria de Souza Lima5 ; Roberta Costa6
; Roberta Costa6 ; Vivian Mara Gonçalves de Oliveira Azevedo7
; Vivian Mara Gonçalves de Oliveira Azevedo7 ; Zeni Carvalho Lamy8
; Zeni Carvalho Lamy8