ABSTRACT
OBJECTIVE: to determine the association between breastfeeding and associated factors with neuropsychomotor development of children living in social vulnerability.
METHODS: cross-sectional study within a socially vulnerable community. Households with children aged seven to 72 months, and their biological mothers were included. Sociodemographic, anthropometric and breastfeeding variables were collected using questionnaires, and neuropsychomotor development was assessed using the Denver II screening test. Adjusted prevalence ratios were calculated using multivariable models, oriented by directed acyclic graphs.
RESULTS: from the 654 households visited, 224 mother-child binomials were included. The mean age of children was 28 (18.7) months, and 143 (63.8%) of them presented suspected delay in neuropsychomotor development. Mothers presented a median of 8 years of formal schooling and 64 (28.6%) had performed exclusive breastfeeding for 6 months. Exclusive breastfeeding was not associated with neuropsychomotor development (PR=0.92; CI95%=0.84-1.00). A significant association was observed only with years of formal maternal education (PR=0.98; CI95%=0.97-0.99). A mediation analysis did not show any clear mediator between maternal education and neuropsychomotor development.
CONCLUSIONS: children living in social vulnerability presented a high prevalence of suspected delay in neuropsychomotor development. Maternal education was the only variable associated with such condition.
Keywords:
Breastfeeding, Child development, Educational status, Child poverty
RESUMO
OBJETIVOS: determinar a associação entre o aleitamento materno e fatores associados ao desenvolvimento neuropsicomotor de crianças em extrema vulnerabilidade social.
MÉTODOS: estudo transversal conduzido em uma comunidade em vulnerabilidade social, envolvendo crianças de sete a 72 meses, e suas mães biológicas. Variáveis sociodemográficas, antropométricas e de amamentação foram coletadas por meio de questionários e o desenvolvimento neuropsicomotor foi avaliado por meio do teste de triagem Denver II. Razões de prevalência ajustadas foram calculadas usando modelos multivariáveis, orientados por grafos acíclicos direcionados.
RESULTADOS: dos 654 domicílios visitados, foram incluídos 224 binômios mãe-filho, com média de idade de 28,8 (18,7) meses, em que 143 (63,8%) crianças apresentavam suspeita de atraso no desenvolvimento neuropsicomotor e 64 (28,6%) haviam realizado aleitamento materno exclusivo até o sexto mês. Aleitamento materno exclusivo por 6 meses não se associou ao desenvolvimento neuropsicomotor (RP= 0,91; IC95%=0,83-1,00). Houve associação significativa observada apenas com anos de escolaridade materna formal (RP=0,97; IC95%=0,96-0,99). Análise de mediação não mostrou nenhum mediador entre escolaridade materna e desenvolvimento neuropsicomotor.
CONCLUSÕES: destaca-se a alta prevalência de crianças com suspeita de atraso no desenvolvimento neuropsicomotor. A escolaridade materna foi a única variável associada à esta condição.
Palavras-chave:
Aleitamento materno, Desenvolvimento infantil, Escolaridade, Pobreza infantil
IntroductionThe first 1000 days of life, a period that extends approximately between conception and the second year of life, is considered a unique period of opportunity, as it establishes the foundations of ideal neurological health, growth, and development throughout life.
1 For these reasons, this period can also be considered of higher vulnerability, whereas, in the presence of a favorable environment, a primary caregiver, and a healthy diet, the central nervous system (CNS) normally develops.
2 Although neurodevelopment continues throughout the life of a healthy person, at two years of age, the CNS undergoes intense restructuring, however, limited to this period, as it will not occur in the later stages of life.
3The child's development during early childhood is influenced by numerous factors linked to maternal aspects, which include the practice of smoking,
4 maternal education,
5 and inadequate nutrition, specifically breastfeeding.
6 Exclusive and complementary breastfeeding time, in the short term, determines verbal evolution,
7 higher aptitude for motor activities,
8 and in the long term, affects cognitive development, strongly influencing the educational performance.
9,10 Therefore, the World Health Organization recommends that breastfeeding should be carried out exclusively until the child is six months old, contributing more significantly to its growth and good development.
11 The positive effects of breast milk may be due to its profile in essential fatty acids, which includes docosahexaenoic acid and arachidonic acid, which are essential in the development of the CNS.
12Thus, a growing number of studies have sought to assess neuropsychomotor development (NPMD); however, it is clear that, especially in clinical practice, there is no standardization of the instruments that should be used to screen child NPMD. Thus, the American Academy of Pediatrics published in 2006 the most recommended screening tests for use in clinical practice, among which is the Denver II.
13 The Denver-II screening test proves to be the most feasible multidimensional test for measuring child NPMD and has already been validated in several countries around the world, including Brazil.
14 Furthermore, the 2015 Sustainable Development Goals
15 require new research and interventions to prioritize solutions to the global challenge of deficient child development in low and middle-income countries,
16 once poverty and adverse childhood experiences, such as the scarcity of educational, cognitive, economic, or health resources, have long-term physiological and epigenetic effects on brain development and cognition.
17Despite the presence of studies with children in social vulnerability, they are associated only with cognitive function, and studies that evaluate NPMD do not exclusively address children in extreme vulnerable conditions.
17,18 Therefore, the objective of this study was to determine the associated factors with the NPMD of children in extreme social vulnerability.
MethodsThis is a cross-sectional study composed of a voluntary sample of mother-child binomials, which took place between March and September 2017. Children aged seven to 72 months and their biological mothers (≥ 16 years), living in communities in the 7
th administrative region of Maceió - AL, Brazil, were included. This region was chosen because it has the lowest Human Development Index (HDI) in the municipality (0.600 - 0.699) and because it presents characteristics of social vulnerability as conceptualized by Souza and Teixeira,
19 which among other factors, present precarious housing, poor access to basic education, a high proportion of inactive family members and consequent economic dependence. The community in which our research was developed contained 634 residences at the time of data collection. Every household was visited and the household chief was asked whether any child lived there. If so, the mother was invited to participate in the research. When there was more than one child in the household, only the data of the youngest child were collected. On the other hand, when there were no children in this age group at home, the neighboring house was visited, until all 634 residences in the community were completed. If the residence was closed, the household was revisited, until confirmation or not of the presence of children in the household. The Brazil Economic Classification Criterion
20 was used to obtain the family's socioeconomic profile, and only those households that were in the lowest economic class (D-E) were included in the survey. This classification estimates that people in this lowest stratum have an average monthly household income of 708.19 reais (approximately 125 dollars for the whole household in 2021).
It was previously calculated, using the Fleiss method with continuity correction, the need for 200 individuals, 67 of whom were exclusive breastfeeding (EB) for six months, and 133 without EB for six months. This number was obtained, starting from an expected prevalence of 80% of inadequate NPMD in similar populations.
21 Assuming that EB for six months would be a protective factor, taking this prevalence to 60%, with a risk ratio of 0.75, and assuming that the prevalence of EB for six months in the Brazilian population is around 33%,
22 the results were one individual with EB for six months for every two without EB. The calculations were conducted with the aid of the Epi Info v 7.2.2.2 program (Center for Disease Prevention and Contol, Atlanta, USA) in its StatCalc module.
Two meetings were held to obtain data. The first was through a screening to assess the suitability of the participants to the inclusion criteria. In the second meeting, all the proposed questionnaires were applied. Data collection took place by filling in a standardized and previously tested form. Age (years), sex (female/male), institutionalization (when the child was inserted in some educational institution, daycare center, or school), smoking (yes/no), alcoholism (yes/no), and breastfeeding status of the child were collected. The children's mothers were asked if the children had been breastfed at some point in their lives. Those who responded positively were asked whether this breastfeeding occurred only in the maternity ward or continued at home. Those who answered that it was only at the maternity ward, and that when they arrived at home, breast milk was no longer offered, it was considered that the child was not breastfed. Those who remained at home were asked until what age the child received exclusive breastfeeding, without receiving water/tea/milk or any other food. It was also questioned until what age the child was breastfed in a complementary way, that is, after starting to introduce food.
Children under two years old were weighed on a scale with a capacity of 15 kg and accurate to five grams (Tecline
®, São Paulo, SP). For those over two years old, and mothers, body mass was measured on a scale (Tecline, São Paulo, SP) with a capacity of 150 kg with 100g precision, all previously calibrated. An infantometer (Alturaexata, Belo Horizonte, Minas Gerais) equipped with an inextensible measuring tape measuring 105 cm in length and with a 0.1 cm accuracy was used to measure the length of children under two years old. A stadiometer (Alturaexata, Belo Horizonte, Minas Gerais) was used to measure the height of those over two years old, equipped with an inextensible measuring tape with a length of 2 m and precision of 0.1 cm. Children were weighed and measured barefoot and wearing light clothing. The weight-for-age, height-for-age, and body mass index (BMI)-for-age indices and the reference curves of the World Health Organization, with the aid of the AnthroPlus software, were used to classify the children's nutritional status.
23 Height was classified using the height-for-age index, in which children with height-for-age < -2 Z score were considered to be stunted. For children aged zero to five years old, those with weight-for-age < -2 Z score were considered underweight. Concerning the mother, the following variables were collected: age, age at which she became pregnant, education (in years of formal schooling), smoking, and alcohol consumption.
The Denver II screening test was used to assess child NPMD.
24 The test consists of 125 items and assesses four areas of function: Personal-social: living with people and taking care of personal needs; Fine-adaptive motor skills: eye-hand coordination, manipulation of small objects, and problem-solving; Language: hearing, understanding, and use of language; Gross motor: sit, walk, jump and large global muscle movement. Furthermore, five "test behavior items for completion after test administration" are included to help the viewer subjectively assess the child's overall behavior and obtain an approximate indicator according to the child's ability. Each test task can be classified as "delay" in case the child is not able to develop it; "Alert" when the child performs the task, but not correctly and "satisfactory" when the child performs the task properly.
The test result was used as a measure on a continuous scale,
25 this method considers that if a child obtains a maximum of two scores with "alert" and none with "delay" it is considered to have normal NPMD. On the other hand, if it obtains two or more "alert" scores and/or one or more scores like "delay", it is considered as "risk of delay in the NPMD". In this study, children classified as "risk of delay in the NPMD" were considered to have a suspected delay in NPMD. All tests were applied by a single physiotherapist professional, with adequate and specific training, in order to avoid possible interpretation bias. The average test application time was 20 minutes.
As part of the project, all families received guidance about the stages of child development, and workshops on ways to encourage child development. The results of the study were forwarded to the basic health unit in the community, and families with suspected developmental delay were instructed to seek the Basic Health Unit.
All analyses were performed using the JAMOVI software (The Jamovi Project, v 4.2, Sidney, Australia). All categorical variables are presented as frequencies and continuous variables as means and standard deviations. The association between the variables of interest and the outcome studied was estimated with the prevalence ratios (PR), obtained using Poisson regression with robust variance estimation. First, successive univariate regressions were performed. Subsequently, a multivariate model oriented by a Directed Acyclic Graph (DAG) was constructed to identify possible confounders in the relationship between EB for six months (the predictor) and NPMD (the outcome), which included characteristics of the mother (smoking, drinking, age, education, and adolescent pregnancy) and characteristics of the child (institutionalization, and age) as adjustment variables. A DAG mediation was also constructed between maternal education and NPMD. Alpha equal to 5% was considered.
This research was approved by the Research and Ethics Committee of the Federal University of Alagoas (CAAE: 60473216.4.0000.5013).
ResultsSix hundred thirty-four households were visited, and in 224 of these, mothers with biological children met the inclusion criteria. From the 410 households that did not present mothers with biological children that meet the inclusion criteria, 32 were closed at the time of all visits, 135 were not in the lowest economic class according to CCEB and 243 did not have children in the investigated age range. The children included in the study had a mean age of 28.8 with a standard deviation of 18.7 months. Table 1 shows the main socioeconomic and anthropometric characteristics of the included mothers and children, in addition to the result of the Denver II screening test. In the assessment of child NPMD, there were 143 (63.8%) children with suspected delay in NPMD, according to Denver II. Sixty-four (28.6%) children evaluated were in EB for six months. Regarding anthropometric indicators, 24 (10.7%) children were stunted, and only eight (3.6%) were underweight. Twenty-six (11.6%) mothers were under 19 years old, and 135 (60.3%) reported having less than eight years of schooling.
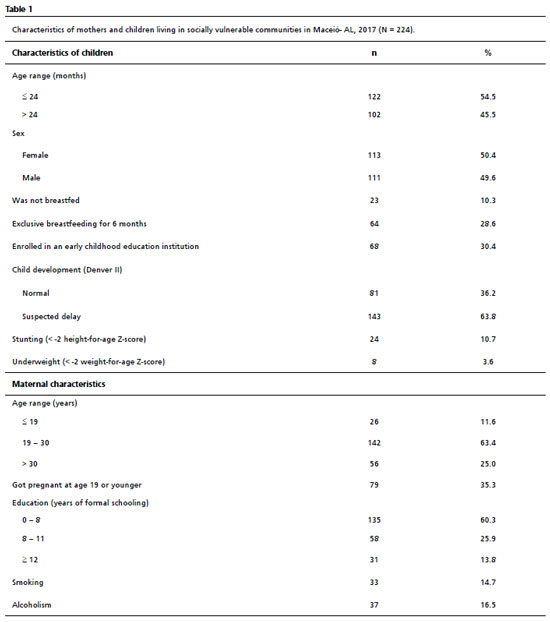
The univariate regression analysis (Table 2) showed a significant association between maternal education, underweight, and EB for six months with suspected delay in NPMD. The DAG oriented analysis (Figure 1), after the adjustments for confounding, indicated that maternal education remained significant in the multivariate analysis (PR= 0.98; CI95%=0.96-0.99;
p<0.01), whereas EB for six months did not maintained statistical significance (PR=0.91; CI95%=0.83-1.00;
p=0.06 (Table 2). In a model including only possible mediators between maternal education and NPMD (that is, EB, stunting, underweight, mother's smoking, mother's alcohol use, and adolescent pregnancy, Figure 2), the maternal education in years remained highly significant as a determinant of children NPMD (PR=0.97; CI95%=0.95-0.98;
p<0.01).
DiscussionThis study investigated children living in the slums of Maceió, an area with a low HDI, and found a prevalence of 63.8% of suspected NPMD delay in these children, according to Denver II, suggesting that the situation of social vulnerability may be associated with the deficit of childhood NPMD. Such prevalence of suspected delay in NPDM is higher than estimates that indicate that 43% of children under five years of age living in low- and middle-income countries would present some risk of not reaching their full physical, cognitive, psychological and social emotional potential.
16 Furthermore, it was also found that the suspected delay in NPMD was not associated with EB for six months, in the multivariate analysis, in which the only determinant of the suspected delay in NPMD was low maternal education.
Although an association between EB and the suspected delay in NPMD was observed only in the univariate analysis, other studies have observed a dose-response effect about the duration of breastfeeding. On which, the longer the breastfeeding time, the lower the risk of a Denver II screening test with suspected delay, providing some evidence that prolonged and EB improves children's cognitive development.
26 In Brazil, the results of a prospective birth cohort indicated a significant cognitive advantage, associated with breastfeeding, in adult individuals, at the age of 30.
10 The study of the relationship between breastfeeding and children's intelligence/mental development is focused on the duration of this practice, since it depends on the effect of polyunsaturated fatty acids resulting from breastfeeding.
27 Nevertheless, it is notable that the change in approach to breastfeeding in our study through EB until the sixth month of age showed a borderline protective association in the adjusted analyses and in the crude analyzes.
Evidence shows that children from the most impoverished families perform poorly on most child NPMD measures.
5 However, the prevalence of suspected delay in NPMD ranged from 2.8% to 35.8% in the lowest socioeconomic levels of other studies that analyzed the child NPMD of Brazilian children.
16,18 These studies, which were not conducted specifically in populations in situations of extreme social vulnerability, but contemplated samples with similar age range, may have shown a lower prevalence than the present study because they investigated a more heterogeneous sample regarding the socioeconomic condition.
16 It should be noted that as the sample in the present study is composed only of people in the lowest income strata, EB should be the only food alternative for some children under six months of age, since the purchase of infant formula becomes inaccessible. However, what we observed is that only 28.6% adhered to the recommendation of the World Health Organization. This may have hindered us in finding an association with the suspicion of delay in NPMD.
New estimates, based on variables associated with children's development such as stunting and extreme poverty, indicate that 250 million children under five-years-old in low and middle-income countries are at risk of not reaching their development potential.
16 From this perspective, it is understood that the socioeconomic status of childhood, characterized by education, occupation, and income of parents, is associated with early experiences essential for cognitive development. In our analysis, low maternal education was the only determinant of the suspected delay in NPMD. This association has already been reported by other authors who identified the worst performances in the domains observed by Denver II in children from the poorest families and children of mothers with less education.
5 Casale and Demond,
28 when analyzing the implications for the cognitive function of stunting recovery in early childhood, found that maternal education had a significant effect. However, when including variables that represent the home environment and caregiver data in its model, significance in the association was not maintained, suggesting that the positive effect of the mother's education on cognitive function operates through some of these other variables.
Low maternal education is a variable used to reflect the socioeconomic risk.
16 The model of systems that form the basis of our structure throughout life includes an enabling environment for the family, community and, especially, for the caregiver, in which it is represented not only by the mother's physical and mental health but also by maternal education.
16 The education of family members, specifically mothers, is related to better family cost management in childcare and public services to the family since education contributes significantly to the improvement of family income and of the population's living and health conditions generally.
29The present study has some limitations: first, the participants' recall bias during the collection of some variables, such as the EB period, can compromise the variable's temporal characteristic because it is a self-reported variable, which depends on the participants' memory, considering that breastfeeding usually occurs in times of stress and sleep deprivation, in which the memory of past events can be especially prone to inaccuracies. Second, the cross-sectional design prevents the attribution of causality in the association between variables. Regarding the use of the Denver II test used in our study, it does not determine whether the child has an inadequate NPMD, but indicates the presence of a risk of child NPMD delay or a suspected delay in NPMD, as reported. Furthermore, this tool is a very crude measure of child development, which may be subject to misclassification, leading to reduced statistical power to detect associations. Despite the various criticisms that the DENVER II tool has received over the years, in which its use is often discouraged due to its specificity, it presents reliable data regarding sensitivity (83%) and reliability (0.80 - 0.90), which validates our use in the present study.
30 Thus, we still suggest caution in the interpretation of data referring to this scale. In addition, we highlight the fragility of the questions used to assess smoking and alcoholism and the absence of data regarding the child's birth (prematurity, birth weight), race/skin color, primary caregiver of the child, domestic violence and excessive use of screens. Another limitation that should be taken into account is related to the way in which exclusive breastfeeding was questioned, which may reduce our comparability with other studies. However, such questioning was carried out trying to adapt to the reality of the participating mothers. Finally, it should be noted that the contribution of the EB on the NPMD may not be evidenced due to the small number of children in this classification of EB up to the sixth month of age.
In conclusion, there is a high prevalence of children with suspected delay in NPMD in this group of children living in extreme social vulnerability. The association with maternal education stands out. These factors motivate the development of new investigations, including interventions that encourage accessible resources for the progression of maternal education and the creation of new protocols for the generation of infant stimuli to generate adults with higher productive ability.
References1. Cusik SE, Georgieff MK. The Role of Nutrition in Brain Development: The Golden Opportunity of the "First 1000 Days". J Pediatr. 2016; 75: 16-21.
2. Georgieff MK, Brunette KE, Tran PV. Early life nutrition and neural plasticity. Dev Psychopathol. 2015; 27 (2): 411-23.
3. Fox SE, Levitt P, Nelson CA. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010; 81 (1): 28-40.
4. Polanska K, Krol A, Merecz-Kot D, Ligocka D, Mikolajewska K, Mirabella F,
et al. Environmental tobacco smoke exposure during pregnancy and child neurodevelopment. Int J Environ Res Public Health. 2017; 14 (7): 796.
5. Boo FL, Mateus MC, Duryea S. Analysis of socioeconomic gradients in the development of children aged 0–3 years in Fortaleza, Northeastern Brazil. Rev Saúde Pública. 2018; 52.
6. Darling AL, Rayman MP, Steer CD, Golding J, Lanham-New S, Bath SC. Association between maternal vitamin D status in pregnancy and neurodevelopmental outcomes in childhood: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Br J Nutr. 2017; 117 (12): 1682-92.
7. Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gadian DG, Lucas A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res. 2010; 67 (4): 357-62.
8. Santos MM, Corsi C, Marques LA, Rocha NA. Comparison of motor and cognitive performance of children attending public and private day care centers. Braz J Phys Ther. 2013; 17 (6): 579-87.
9. Relvas GR, Buccini G, Potvin L, Venancio S. Effectiveness of an educational manual to promote infant feeding practices in primary health care. Food Nutr Bull. 2019; 40 (4): 544-61.
10. Victora CG, Horta BL, Mola CL, Quevedo L, Pinheiro RT, Gigante D,
et al. Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: a prospective birth cohort study from Brazil. Lancet Glob Health. 2015; 3 (4): e199-205.
11. World Health Organization (WHO). Infant and young child feeding. [
Internet]. [access in 2021 Oct 28]. Available from:
https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding12. McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology, prostaglandins. Leukot Essent Fatty Acids. 2006; 75 (4-5): 329-49.
13. Council on Children With Disabilities, Section on Developmental Behavioral Pediatrics, Bright Futures Steering Committee, and Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics. 2006 Jul; 118 (1): 405-20.
14. Rubio-Codina M, Araujo MC, Attanasio O, Muñoz P, Grantham-McGregor S. Concurrent validity and feasibility of short tests currently used to measure early childhood development in large scale studies. PLoS One. 2016; 11 (8): e0160962.
15. United Nations. Take action for the Sustainable Development Goals. [
Internet]. [access in 2020 Jul 10] Available from:
https://www.un.org/sustainabledevelopment/sustainable-development-goals/16. Black MM, Walker SP, Fernald LC, Andersen CT, DiGirolamo AN, Lu C,
et al.; Lancet Early Childhood Development Series Steering Committee. Early childhood development coming of age: science through the life course. Lancet. 2017 Jan; 389 (10064): 77-90.
17. Vargas T, Damme KS, Mittal VA. Neighborhood deprivation, prefrontal morphology and neurocognition in late childhood to early adolescence. Neuroimage. 2020 Oct; 220: 117086.
18. Halpern R, Barros AJ, Matijasevich A, Santos IS, Victora CG, Barros FC. Developmental status at age 12 months according to birth weight and family income: a comparison of two Brazilian birth cohorts. Cad Saúde Pública. 2008; 24 (Suppl.): S444-50.
19. Souza MI, Teixeira KH. Uma análise espacial da vulnerabilidade social em Alagoas: evidências sobre as mulheres e crianças. Econ Soc Territ. 2019; 19 (61): 451-77.
20. Associação Brasileira de Empresas de Pesquisa (ABEP). Critério de classificação econômica Brasil. [
Internet]. [access in 2020 Jul 10]. Available from:
http://www.abep.org/criterio-brasil/21. Saccani R, Brizola E, Giordani AP, Bach S, Resende TL, Almeida CS. Avaliação do desenvolvimento neuropsicomotor em crianças de um bairro da periferia de Porto Alegre. Sci Med. 2007; 17 (3): 130-7.
22. Boccolini CS, Boccolini PM, Monteiro FR, Venâncio SI, Giugliali ER. Tendência de indicadores do aleitamento materno no Brasil em três décadas. Rev Saúde Pública. 2017; 51: 1-9.
23. World Health Organization (WHO). WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: WHO; 2006. [access in 2020 Jul 10]. Available from:
https://www.who.int/publications/i/item/924154693X24. Frankenburg WK, Dodds JB, Fandall AW. Denver Developmental Screening Test: Manual. Ed. Revised. Denver: University of Colorado Medical Center; 1970.
25. Drachler ML, Marshall T, Leite JC. A continuous-scale measure of child development for population-based epidemiological surveys: a preliminary study using item response theory for the Denver Test. Paediatr Perinat Epidemiol. 2007; 21 (2): 138-53.
26. Halpern R, Giugliani ER, Victora CG, Barros FC, Horta BL. Fatores de risco para suspeita de atraso no desenvolvimento neuropsicomotor aos 12 meses de vida, J Pediatr. 2000; 76 (6): 421-8.
27. Bernard JY, Armand M, Peyre H, Garcia C, Forhan A, De Agostini M,
et al. Breastfeeding, polyunsaturated fatty acid levels in colostrum and child intelligence quotient at age 5-6 years. J Pediatr. 2017; 183: 43-50.e3.
28. Casale D, Desmond C. Recovery from stunting and cognitive outcomes in young children: evidence from the South African Birth to Twenty Cohort Study. J Dev Orig Health Dis. 2016; 7 (2): 163-71.
29. Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Sociol. 2010; 36: 349-70.
30. Santos JAT, Ayupe KMA, Lima ALO, De Albuquerque KA, Morgado FFR, Gutierrez Filho PJB. Propriedades psicométricas da versão brasileira do Denver II: teste de triagem do desenvolvimento. Ciênc Saúde Colet. 2022; 27 (3): 1097-106.
Author's contribution: Camilo LS: Study design, Data collection, Manuscript writing; Bueno NB: Data analysis, Manuscript review; Macena ML: Data analysis, Manuscript review; Silva-Neto LGR: Data analysis, Manuscript writing; Britto RPA: Manuscript review, Study supervision; Silva MEN: Data collection; Florêncio TMMT: Study design, Manuscript review, Study supervision. All authors approved the final version of the article and declare no conflict of interest.
Received on February 16, 2023
Final version presented on November 27, 2023
Approved on December 27, 2023
Associated Editor: Priscilla Onofre
 ; Nassib Bezerra Bueno2
; Nassib Bezerra Bueno2 ; Mateus de Lima Macena3
; Mateus de Lima Macena3 ; Luiz Gonzaga Ribeiro Silva-Neto4
; Luiz Gonzaga Ribeiro Silva-Neto4 ; Revilane Parente de Alencar Britto5
; Revilane Parente de Alencar Britto5 ; Maria Edislândia Nunes da Silva6
; Maria Edislândia Nunes da Silva6 ; Telma Maria de Menezes Toledo Florêncio7
; Telma Maria de Menezes Toledo Florêncio7