ABSTRACT
OBJECTIVES: to estimate the proportion of seroconversion cases among infants exposed to HIV and verify the risk factors associated.
METHODS: this was a historical cohort study conducted in the State of Santa Catarina between 2007 and 2017. The data were obtained from the Notifiable Diseases Information System (SINAN - Portuguese acronym) that records HIV-infected pregnant women and HIV-exposed infants. The public health service monitored the infants from birth to 18 months of age to determine whether HIV seroconversion occurred.
RESULTS: a total of 5,554 HIV-infected pregnant women were included in the study (mean age 26.7±6.5 years). They were predominantly white, with poor education level, and were diagnosed with HIV until the 2nd trimester of pregnancy. A total of 4,559 records of HIV-exposed infants were screened, of which 130 cases (2.9%) of seroconversion were confirmed. Non-use of antiretroviral drugs during pregnancy (OR=9.31, CI95%=5.97-14.52; p<0.001) and breastfeeding (OR=3.10, CI95%=1.34-7.20; p=0.008) were independent risk factors for seroconversion.
CONCLUSIONS: these data demonstrate gaps in prenatal care, regarding adherence to treatment and monitoring of HIV-infected mothers, resulting in new cases of HIV among children, which could be avoided.
Keywords:
HIV, Acquired Immunodeficiency Syndrome, Seroconversion, Infectious disease transmission, Vertical, Risk factors
RESUMO
OBJETIVOS: estimar a proporção de soroconversão da criança exposta ao HIV e verificar os fatores de risco associados, no período de 2007-2017 em Santa Catarina.
MÉTODOS: o delineamento utilizado foi de coorte histórica e os dados obtidos no Sistema de Informação de Agravos de Notificação (SINAN) que registra as gestantes infectadas e as crianças expostas ao HIV. As crianças foram acompanhadas pelo serviço de saúde desde o nascimento até o 18º mês de vida, para determinar a ocorrência de soroconversão pelo HIV.
RESULTADOS: foram identificadas 5.554 gestantes infectadas pelo HIV com média de idade de 26,7±6,5 anos, predomínio da raça branca, baixa escolaridade e que receberam o diagnóstico para o HIV até o 2º trimestre gestacional. Foram incluídas 4.559 fichas de crianças expostas ao HIV, das quais 130 casos (2,9%) de soroconversão foram confirmados. O não uso de antirretroviral durante a gestação (OR=9,31, IC95%=5,97-14,52; p<0,001) e aleitamento materno (OR=3,10, IC95%=1,34-7,20; p=0,008) foram fatores de risco independentes para a soroconversão.
CONCLUSÕES: esses dados demonstram lacunas na assistência pré-natal, quanto a adesão ao tratamento e acompanhamento de mães infectadas pelo HIV, resultando em casos novos de HIV entre crianças, que poderiam ser evitados.
Palavras-chave:
HIV, Síndrome de imunodeficiência adquirida, Soroconversão, Transmissão vertical de doença infecciosa, Fatores de risco
IntroductionHIV infection affects women of reproductive age,
1 and worldwide, most pregnancies in infected women are unintentional.
2 More than 90% of the incident cases of HIV infections in children are attributed to mother-to-child transmission.
3 It may occur in the gestational, intrapartum and postpartum periods during breastfeeding.
4 According to data from the Brazilian Ministry of Health (MH), between 2000 and 2019 there were 125,144 HIV-infected pregnant women, resulting in a rate of 2.9/thousand live births. In the state of Santa Catarina (SC) there was a total of 8,642, with a rate of 6.1/thousand live births in the same period.
5To achieve the goals of preventing maternal HIV transmission, it is essential to extend hiv screening testing and antiretroviral therapy (ART) testing for infected pregnant women.
6 Therefore, prenatal and pharmacological treatment during pregnancy in cases of HIV infection, when peak transmission periods are high, are essential factors to suppress viral load (VL) as much as possible and prevent vertical transmission.
7 Studies suggest that HIV-infected children have high mortality rates, infectious morbidity and impaired growth compared to unexposed children.
8In Brazil, the MH recommends HIV testing for all pregnant women in the first and third trimesters of pregnancy, compulsory registration of positive cases among pregnant women and children exposed to the risk of transmission, in addition to other recommendations in the peripartum that aim to prevent the seroconversion of the child.
7 Access to antiretroviral drugs is available through prenatal services and maternity hospitals, and breastfeeding is not recommended among HIV-infected parturient.
7.9Several factors may be associated with vertical transmission and seroconversion of HIV infection in children, such as socioeconomic factors, ignorance of serological status, elevated VL, vaginal delivery and breastfeeding.
10,11 The late diagnosis of HIV infection and non-adoption of ART are factors that may contribute to the child’s seroconversion.
4,12 Thus, in order to eliminate vertical transmission of HIV, it is essential to identify and control the factors that contribute to the viral transmission chain.
13Even with interventions to prevent vertical transmission of HIV, it is still a reality. Between 2008 and 2018, there was an increase in 38.1% of cases of infected pregnant women,
5 which points to the potential risk of vertical transmission. However, data from newborns of HIV-infected mothers are scarce, justifying the present study.
The aim of this study is to estimate the proportion of seroconversion of children exposed to HIV in SC in the period between 2007 and 2017, and the associated risk factors.
MethodsEpidemiological study with a historical cohort design. A survey of children (0-18 months) exposed to HIV who were born between January 1, 2007 and December 31, 2017, in SC, was conducted. Data were collected from the Notifiable Diseases Information System (SINAN) of the MS from the Investigation Forms of HIV+ pregnant women and Children Exposed to HIV. The children were followed by the health service from birth to the 18th month of life to determine the occurrence of HIV seroconversion. The individualized data, however, being anonymous, were provided by the Directorate of Epidemiological Surveillance of CS. Children were considered Hiv-infected when defined so in the case evolution, in the closing of the diagnostic criterion, besides DEATHS related to HIV/AIDS recorded in the SINAN notification form.
The Protocol of MS
14 in force during the study period determined that all pregnant women infected with HIV were notified, and the case was updated regarding the gestational outcome (live birth, stillbirth, or abortion). In the case of children exposed to HIV, they would be notified at birth, and the diagnosis of seroconversion was made by means of two VL tests. Children are considered as having no indication of infection when there are two consecutive results of undetectable VL, and infected if there are two consecutive VL results above 5,000 copies/ml. It is recommended to perform HIV serology in children 18 months and older. Therefore, municipal health services are able to update the same notification, from the follow-up of children exposed to HIV up to this age. Once seroconversion is confirmed, HIV-infected children are notified.
14All pregnant women diagnosed with HIV infection and vertical exposure of their children between 0 and 18 months, of both sexes, living in SC and notified in SINAN during the study period, were included in the study. Maternal, pregnancy, delivery and peripartum sociodemographic data were collected, and child-related data were collected in the respective compulsory notification forms.
The outcome of this study was the confirmation of seroconversion during the follow-up period of the child (up to 18 months of age). It was considered as seroconversion the infected children and those who died with mention to aids, HIV infection or inconclusive investigation in the death certificate according to the determination of the MH Protocol,
14 as recorded in SINAN. The following maternal variables were studied: age (in years, grouped into age groups 12-19, 20-29, 30-39, 40-49 and ≥50 years), race/skin color (white, black, brown, indigenous, yellow), schooling (unliterate, 0-8 and >8 years of schooling), and area of residence (rural, urban or peri urban); factors associated with vertical transmission: whether attended prenatal care (yes or no), diagnosis (1st, 2nd or 3rd trimester), use of ART during pregnancy and at the time of delivery (yes or no), type of delivery (vaginal or cesarean section), evolution of pregnancy (abortion, stillbirth or live birth) and use of ART by the newborn (yes or no). Variables related to children were also included: gender (male or female), race/skin color (white, black, brown, indigenous, yellow), oral prophylaxis (yes or no), time of use of postpartum ART (in weeks), breastfeeding and cross feeding (yes or no) and outcome of the case (infected, not infected, death from AIDS or other causes, probable non-infection, transfer, in progress or loss of follow-up). The data not found in the records were presented as ignored.
The digital databases (of pregnant women and children exposed to HIV) obtained were exported to IBM SPSS Statistics
® software, version 21 (IBM
®, Armonk, New York, USA). Descriptive analyses were performed, and categorical variables were expressed in proportions and numerical variables expressed as mean and standard deviation (SD). The Kolmogorov-Smirnov test was applied to verify the normality of quantitative variables. The proportion of seroconversion was calculated by the number of HIV infection cases confirmed in relation to the total number of children exposed in pregnancy x 100.
To verify the risk factors for HIV seroconversion in children born to infected mothers, the risk estimate was calculated by odds ratio (OR), with confidence interval (CI) of 95%. To adjust the confounding factors, multiple logistic regression was used by the Enter method
, including in the model all variables that presented
p<0.20 value in the bivariate analysis. The level of statistical significance adopted in this study was 5% (
p<0.05).
This study was approved by the Research Ethics Committee (CEP) of the University of Southern Santa Catarina under opinion no. 3,137,377, CAAE 03393418.5.0000.5369 on February 8, 2019.
ResultsA total of 5,554 HIV-positive pregnant women were analyzed. Table 1 presents the characteristics of mothers living with HIV and data on gestational follow-up. There was a predominance of young women (up to 29 years), with a mean age of 26.7 years (SD±6.5), white, low schooling (up to eight years of schooling), residents in urban areas, who underwent prenatal follow-up, received a diagnosis for HIV until the 2nd trimester of pregnancy, used antiretroviral therapy during pregnancy and childbirth, with the outcome of children born alive and who received antiretroviral prophylaxis in the first few years 24 hours after birth.
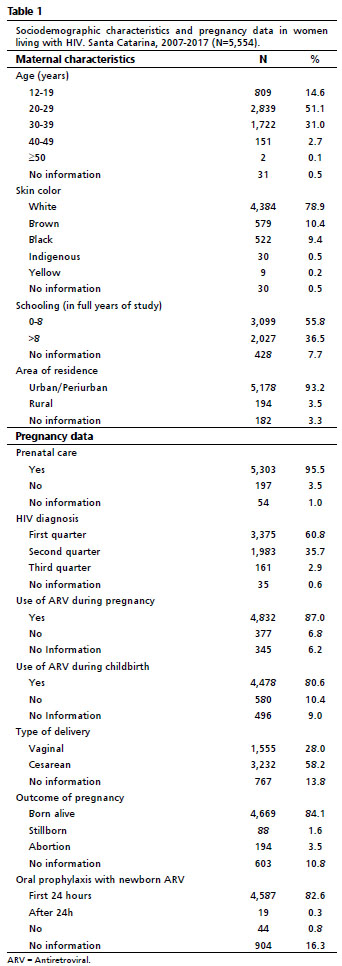
We included 4,559 records of children exposed to HIV, that is, born to mothers living with HIV. Table 2 shows the demographic and prophylaxis characteristics for HIV infection vertically. There was a predominance of white children (79.8%) born by cesarean section (66.3%), who received oral antiretroviral prophylaxis in the peripartum for six weeks (64.9%) and did not receive breastfeeding or cross-feeding (94.4%).
Seroconversion occurred in 2.9% of the exposed children. Table 3 presents the results of the association analyses for the investigation of risk factors for seroconversion. It was observed that the non-use of antiretroviral during pregnancy (OR=9.31, CI95%=5.97-14.52;
p<0.001) and breastfeeding (OR=3.10, CI95%=1.34-7.20;
p=0.008) were independent risk factors for the occurrence of HIV seroconversion. The use of antiretroviral drugs in childbirth (
p=0.092), the type of delivery (
p=0.247) and the use of antiretroviral drugs by the newborn after birth (
p=0.923) were not factors associated with the occurrence of seroconversion for HIV infection in this study (Table 3).
DiscussionIn the sample studied, seroconversion was confirmed in 2.9% of the cases, being associated with breastfeeding and non-use of antiretroviral during pregnancy. This may be due to risky behaviors or lack of knowledge of the mothers of infected children, such as the difficulty of adhering to pharmacological treatment and negligence regarding prophylactic care.
5The demonstrated low schooling of pregnant women living with HIV corroborates with other studies conducted in Brazil and worldwide,
4,15-17 that showed low level of education in this portion of the population. The level of education is an important indicator analogous to socioeconomic variables
17,18 and low schooling contributes to the non-assimilation of information, hindering the adhering to prevention practices, increasing the risk of vertical transmission.
4,19 Regarding age group, similar to other findings,
15,17,20 the results show a higher percentage of cases in young adult women. In Brazil, the age group most affected by HIV/AIDS in pregnant women is between 20 and 24 years
21 corresponding to women, in general, at the beginning of reproductive age, which reinforces the need for attention to this public in order to reduce the risk of vertical transmission.
22 This reality is also evidenced in African countries, especially in Sub-Saharan Africa.
9Regarding race, white was the most prevalent with 78.9% of women, corroborating Brazilian data that show the highest number of HIV/AIDS cases among the white group of people.
5 This reality contrasts with international data, since HIV is more prevalent among black people, who are sometimes more vulnerable because of their socio-economic status.
23 It is a subject to reflect about whether these data may be influenced by social inequality in relation to race, in which black and brown women may have less access to HIV testing and care in health services. However, data from the Brazilian Institute of Geography and Statistics (IBGE – Portuguese acronym)
24 indicate that the majority of the Brazilian population declares themselves white, especially in the State of Santa Catarina, due to the colonization of European descendants in the various regions of the State.
The results of this study reinforce the importance of prenatal care for the early diagnosis of HIV infection with a view to timely treatment, since most women were diagnosed in the first trimester of pregnancy. Similar data were found in studies set at the municipalities of Santa Maria (RS)
17 and Belo Horizonte (MG).
25 Women may have a persistent risk of HIV infection in pregnancy and postpartum, which is why there is a recommendation for testing in the first and third trimester of pregnancy, and HIV testing during breastfeeding is recently recommended.
26 Early diagnosis is essential for the prevention of vertical HIV transmission, as it allows the implementation of clinical measures to reduce mortality and disease progression.
27Late diagnosis and ignorance of the forms of HIV transmission may contribute to an increase in the number of cases in neonates, especially due to high maternal viral load.
26 And it is estimated that the screening of cases reaches only 58.3% of the expected cases of HIV-infected pregnant women, which contributes to the number of cases of vertically infected children.
28Breastfeeding and non-use of ART during pregnancy were risk factors independent of vertical transmission, resulting in a 2.9% proportion of HIV seroconversion. A study in the city of São Luís (MA)
22 also found an association between breastfeeding and HIV infection in children. In line with these findings, a study in southern Santa Catarina
16 concluded that breastfeeding and non-use of antiretroviral drugs during pregnancy were risk factors for HIV seroconversion. In studies conducted in other countries, it is also observed evidence of the non-adherence to the protocol of prophylaxis of vertical transmission between factors associated with vertical transmission, especially when maternal infection occurs during pregnancy or in the postpartum period.
4.25These data reflect the lack of follow-up of mothers to the protocol recommended for the prophylaxis of vertical transmission, which demonstrates failures in the process of capture and active search of pregnant women who miss prenatal consultation.
16 In 2017, in Brazil, 23.2% of HIV-infected women had insufficient treatment and 9% were in a situation of ART abandonment. These data represent a total of 48,476 women who did not have adequate support and 18,898 who abandoned treatment.
29 Thus, as well as identifying viral infection and initiating ART early, it is important that women be linked and retained to health services so that they have the maximum benefit of care.
12 Low-adherence or abandoning antiretroviral treatment was also observed in other countries, both in the gestational and postpartum periods.
3Knowledge of the profile of HIV-infected pregnant women is fundamental for the development of more effective strategies to control vertical transmission, in addition to evaluating the quality of the health system and determining the vulnerability of women.
13 The elimination of vertical HIV transmission was established as a national priority for the years 2019 and 2020, with the goal of reducing the number of HIV cases in children to less than 2% or reducing it to 0.
30Among the limitations of the study is the possibility of underreporting or delays in notification. It should be considered that women with greater social vulnerability may not have had access to services even for HIV diagnosis, nor for adequate follow-up of the case. Once this is a retrospective cohort study using secondary databases, with a large percentage of ignored data, the conclusions should be analyzed with caution. In addition, the anonymity of the databases prevented the monitoring the evolution of cases since pregnancy and the consultation of data from the Laboratory Test Information System and the Logistic Control System of Medicines to verify viral load, maternal immunity, and treatment adherence.
Based on the findings of the present study, more than 4,500 children born in SC from 2007 to 2017 were vertically exposed to HIV and seroconversion was confirmed in 2.9% of cases. Seroconversion was associated with non-use of antiretroviral therapy during pregnancy and breastfeeding.
Considering that there are few studies that analyze the risk factors involved in the seroconversion of neonates, the presented results identified gaps in prenatal care, regarding treatment adhering and postpartum follow-up, and can support the strengthening of policies for assistance to women during pregnancy, childbirth and puerperium, which promote the reduction of vertical transmission and seroconversion of children exposed to HIV.
References1. World Health Organization (WHO). PMTCT strategic vision 2010-2015: Preveting mother-to-child transmission of HIV to reach the UNGASS and Millennium Developement Goals [Internet]. Geneva: WHO; 2010. [access in 2021 Fev 10]. Available from:
http://www.who.int/hiv/pub/mtct/strategic_vision/en/2. Bongomin F, Chelangat M, Eriatu A, Chan Onen B, Cheputyo P, Godmercy SA,
et al. Prevalence and factors associated with contraceptive use among HIV-infected women of reproductive age attending infectious disease clinic at Gulu Regional Referral Hospital, Northern Uganda. Biomed Res Int. 2018 Jun; 2018: 9680514.
3. Omonaiye O, Kusljic S, Nicholson P, Manias E. Medication adherence in pregnant women with human immunodeficiency virus receiving antiretroviral therapy in sub-Saharan Africa: a systematic review. BMC Public Health. 2018 Jun; 18 (1): 805.
4. Belachew A, Tewabe T, Malede GA. Prevalence of vertical HIV infection and its risk factors among HIV exposed infants in East Africa: a systematic review and meta-analysis. Trop Med Health. 2020 Oct; 48: 85.
5. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Boletim Epidemiológico. Número Especial HIV/Aids 2019. [access in 2020 fev 18]. Available from:
http://www.aids.gov.br/pt-br/pub/2019/boletim-epidemiologico-de-hivaids-20196. Schnack A, Rempis E, Decker S, Braun V, Rubaihayo J, Busingye P,
et al. Prevention of Mother-to-Child Transmission of HIV in Option B+ Era: uptake and adherence during pregnancy in Western Uganda. AIDS Patient Care STDS. 2016 Mar; 30 (3): 110-8.
7. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Protocolo clínico e diretrizes terapêuticas para prevenção da transmissão vertical de hiv, sífilis e hepatites virais. Brasília (DF): Ministério da Saúde; 2019. [access in 2020 fev 18]. Available from:
https://portaldeboaspraticas.iff.fiocruz.br/wp-content/uploads/2021/08/miolo_pcdt_tv_08_2019.pdf8. Anderson K, Kalk E, Madlala HP, Nyemba DC, Kassanjee R, Jacob N,
et al. Increased infectious-cause hospitalization among infants who are HIV-exposed uninfected compared to HIV-unexposed. AIDS. 2021 Nov; 35 (14): 2327-39.
9. United Nations AIDS (UNAIDS). Eliminating mother-to-child transmission of HIV and scaling up paediatric care of HIV in western and central Africa [Internet]. 2015. [access in 2020 fev 18]. Available from:
http://www.unaids.org/en/resources/presscentre/featurestories/2015/november/20151125_Dakar10. Succi RCDM. Mother-to-child transmission of HIV in Brazil during the years 2000 and 2001: results of a multi-centric study. Cad Saúde Pública. 2007; 23 (Suppl. 3): S379-89.
11. Pan American Health Organization (PAHO). Elimination of mother-to-child transmission of HIV and syphilis in the Americas. Update. 2016. Washington (DC): PAHO; 2017. [access in 2020 fev 18]. Available from:
https://iris.paho.org/bitstream/handle/10665.2/34072/9789275119556-eng.pdf?sequence=4&isAllowed=y12. Redmond AM, McNamara JF. The road to eliminate mother-to-child HIV transmission. J Pediatr (Rio J). 2015; 91 (6): 509-11.
13. Prado TN, Brickley DB, Hills NK, Zandonade E, Moreira-Silva SF, Miranda AE. Factors Associated with Maternal-Child Transmission of HIV-1 in Southeastern Brazil: A Retrospective Study. AIDS Behav. 2018 Jul; 22 (Supl. 1): 92-8.
14. Ministério da Saúde (BR). Protocolo Clínico e Diretrizes Terapêuticas para Manejo da Infecção pelo HIV em Crianças e Adolescentes. Brasília (DF): Ministério da Saúde; 2018. [access in 2020 fev 18]. Available from:
https://portaldeboaspraticas.iff.fiocruz.br/wp-content/uploads/2019/08/pcdt_infantil_04_2019_web.pdf15. Silva CM, Alves RS, Santos TS, Bragagnollo GR, Tavares CM, Santos AAP. Panorama epidemiológico do HlV/aids em gestantes de um estado do Nordeste brasileiro. Rev Bras Enferm. 2018; 71 (Supl. 1): 613-21.
16. Oliveira KWK, Oliveira SK, Barranco ABS, Hoffmann T, Duarte CS, Nazário RF,
et al. Transmissão vertical do HIV na Região Sul de Santa Catarina, 2005-2015: análise dos fatores de risco para soroconversão em nascidos vivos. Rev Bras Saúde Mater Infant. 2018; 18 (3): 471-9.
17. Konopka CK, Beck ST, Wiggers D, Silva AK, Diehl FP, Santos FG. Perfil clínico e epidemiológico de gestantes infectadas pelo HIV em um serviço do sul do Brasil. Rev Bras Ginecol Obstet. 2010; 32 (4): 184-90.
18. Galvão JMV, Costa ACM, Galvão JV. Demographic and socio-demographic profile of people living with HIV / AIDS. Rev Enferm UFPI. 2017; 6 (1): 4-8.
19. Jordão BA, Espolador GM, Finochio Sabino AMN, Tavares BB. Conhecimento da gestante sobre o HIV e a transmissão vertical em São José do Rio Preto, São Paulo, Brasil. Rev Bras Pesq Saúde. 2016; 18 (2): 26-34.
20. Pimenta A, Duarte G, Couto-Fernandez J, Correa I, Melli P, Quintana S. Gestantes HIV: Características Clínicas e Sociodemográficas. Rev Atenc Saúde. 2015; 13 (45): 20-5.
21. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Programa Nacional de DST e Aids. Recomendações para profilaxia da transmissão vertical do HIV e terapia antirretroviral em gestantes. Brasília (DF): Ministério da Saúde; 2010. [access in 2021 jun 30]. Available from:
https://bvsms.saude.gov.br/bvs/publicacoes/recomendacoes_profilaxia_transmissao_vertical_hiv_5ed.pdf22. Silva MJMS, Mendes WS, Gama MEA, Chein MBC, Veras DS. Perfil clínico-laboratorial de crianças vivendo com HIV / AIDS por transmissão vertical em uma cidade do Nordeste brasileiro. Rev Soc Bras Med Trop. 2010; 43 (1): 32-5.
23. Sullivan PS, Satcher Johnson A, Pembleton ES, Stephenson R,
et al. Epidemiology of HIV in the USA: epidemic burden, inequities, contexts, and responses. Lancet. 2021; 397 (10279): 1095-106.
24. Instituto Brasileiro de Geografia e Estatística (IBGE). População chega a 205,5 milhões, com menos brancos e mais pardos e pretos. [internet]; 2017. [access in 2021 jun 30]. Available from:
https://agenciadenoticias.ibge.gov.br/agencia-noticias/2012-agencia-de-noticias/noticias/18282-populacao-chega-a-205-5-milhoes-com-menos-brancos-e-mais-pardos-e-pretos#:~:text=n%C3%A3o%20estar%20dispon%C3%ADveis.-,Popula%C3%A7%C3%A3o%20chega%20a%20205%2C5%20milh%C3%B5es%2C%20com%20menos%20brancos,e%20mais%20pardos%20e%20pretos&text=Entre %202012%20e%202016%2C%20enquanto,%2C%20totalizando%2090%2C9%20milh%C3%B5es25. Romanelli R, Kakehasi F, Tavares M, Melo V, Goulart L, Aguiar R. Perfil das gestantes infectadas pelo HIV atendidas em pré-natal de alto risco de referência de Belo Horizonte. Rev Bras Saúde Matern Infant. 2006; 6 (3): 329-34.
26. Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV duringPregnancy and Postpartum and Risk of Mother-to-ChildHIV Transmission: A Systematic Review and Meta-Analysis. PLoS Med. 2014 Feb; 11(2): e1001608.
27. Ayala ALM, Moreira A, Francelino G. Características Socioeconômicas e Fatores Associados à Positividade para o HIV em gestantes de uma cidade do sul do Brasil. Rev APS. 2016; 19 (2): 210-20.
28. Rosa MC, Lobato RC, Gonçalves CV, Silva NMO, Barral MFM, Martinez AMB,
et al. Evaluation of factors associated with vertical HIV-1 transmission. J Pediatr (Rio J). 2015; 91 (6): 523-8.
29. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Indicadores e dados básicos de monitoramento clínico de HIV. Brasília (DF): Ministério da Saúde; 2017. [access in 2021 jun 30]. Available from:
http://indicadoresclinicos.aids.gov.br/30. United Nations AIDS (UNAIDS). Transmissão vertical é tema da primeira reunião do GT UNAIDS de 2019. Programa Conjunto das Nações Unidas no Brasil. [internet]; 2019. [access in 2021 jun 30]. Available from:
https://unaids.org.br/2019/08/transmissao-vertical-e-tema-da-primeira-reuniao-do-gt-unaids-de-2019Received on August 12, 2021
Final version presented on March 29, 2022
Approved on March 30, 2022
Authors’ contributionsCunga IVA and Schuelter-Trevisol F did the design and planning of the study. Cunga IVA, Souza BB, Rosa CMA, Iser BPM, Schuelter-Trevisol F were responsible for data collection, analysis and interpretation, preparation, or review of the manuscript. All authors approved the final version of the manuscript, publicly hold themselves responsible for its content and declare that there is no conflict of interest.
 ; Bianca Bittencourt de Souza 2
; Bianca Bittencourt de Souza 2 ; Claudia Maria Augusto da Rosa 3
; Claudia Maria Augusto da Rosa 3 ; Betine Pinto Moehlecke Iser 4
; Betine Pinto Moehlecke Iser 4 ; Fabiana Schuelter-Trevisol 5
; Fabiana Schuelter-Trevisol 5
