ABSTRACT
OBJECTIVES: to investigate the association between prepregnancy body mass index (BMI) and newborns’ (NB) BMI.
METHODS: cohort study with 1,365 pregnant women and their newborns from the BRISA survey (Brazilian Ribeirão Preto and São Luís Birth Cohort Studies) in São Luís-MA. Prepregnancy BMI was self-reported, and newborns’ BMI was estimated using the weight and length measured at birth. A directed acyclic graph (DAG) was developed to identify the adjustment variables. The association between the prepregnancy BMI and newborns’ BMI were analyzed using multiple linear and Poisson regression with robust variance estimation.
RESULTS: NBs had 13.4±1.7kg/m2 average BMI at birth. In the linear analysis, we observed that as the prepregnancy BMI increases, the NBs BMI also increases (ß=0.07; CI95%=0.05–0.09;p<0.001). Newborns of mothers with prepregnancy overweight were 3.58 times more likely to be overweight.
CONCLUSION: prepregnancy BMI can affect newborn’s BMI early. Thus, women planning to become pregnant should consider conducting nutritional planning to maintain or obtain a healthy weight to minimize the risk of overweight for the newborn.
Keywords:
Nutritional status, Body mass index, Pregnancy, Newborn
RESUMO
OBJETIVOS: investigar a associação entre o Índice de Massa Corporal (IMC) pré-gestacional e o IMC do recém-nascido (RN).
MÉTODOS: estudo de coorte, com 1365 gestantes e seus RN, participantes da pesquisa BRISA (Brazilian Ribeirão Preto and São Luís Birth Cohort Studies) em São Luís-MA. O IMC pré-gestacional foi autorreferido e o IMC do RN foi calculado por meio do peso e comprimento aferidos na ocasião do nascimento. Foi elaborado um Gráfico Acíclico Direcionado (DAG)para identificaras variáveis de ajuste. A associação entre o IMC pré-gestacional e IMC do RN foram analisados por regressão linearmúltipla e regressão de Poisson com estimativa robusta da variância.
RESULTADOS: os RN tiveram IMC ao nascer médio de 13,4 ± 1,7 kg/m2. Na análise linear, foi observada que à medida que o IMC pré-gestacional aumenta, o IMC do RN também aumenta (ß= 0,07; IC95%= 0,05 - 0,09; p<0.001). RN de mães com excesso de peso pré-gestacional tiveram risco 3,58 vezes maior de terem excesso de peso.
CONCLUSÃO: o IMC pré-gestacional pode afetar precocemente o IMC do RN. Dessa forma, recomenda-se que mulheres que planejem engravidar considerem realizar um planejamento nutricional para a manutenção ou obtenção de um peso saudável, a fim de minimizar o risco de excesso de peso para o RN.
Palavras-chave:
Estado nutricional, Índice de massa corporal, Gravidez, Recém-nascido
IntroductionAn adequate prepregnancy nutritional status is an essential factor to get a positive outcome in pregnancy and to maintain both mother and child healthy.
1 Its inadequacy is a public health problem sinceit increases the risk of gestational complications and influences fetuses’ health conditions and maternal health in postpartum period.
2,3According to the Committee on the Impact of Pregnancy Weight on Maternal and Child Health,
9 prepregnancy nutritional status is one of the strongest predictors of baby size at birth, along with maternal age, smoking, and parity. Thus, assessing maternal nutritional status is essential not only because it identifies women at gestational risk, but also because of its prognosis on child’s health situation in the first years of life and prevention of perinatal morbimortality.
4-6Investigating the impact of mother’s nutritional status on their newborns, some studies evaluated the association between prepregnancy BMI and newborn’s birth weight.
7–9 However, we lack studies evaluating newborn’s BMI at birth as an outcome. Body mass index (BMI) is an important indicator of nutritional status with cutoff point in all age groups, enabling to follow individuals with the same indicator throughout their entire life, without needing to transition between different measures.
10,11Unlike birth weight measurement alone, BMI measures the proportionality between weight and height (or length).
12 Despite its measurement limitations, BMI is also used as an indirect indicator of adiposity, as it has a good correlation with gold standard methods.
13,14Studies estimate that 21.7% to 41.7% of overweight and obesity in childhood is attributed to their mothers’ prepregnancy overweight.
15 Studies that analyzed individuals’ BMI throughout their life showed that overweight children are more likely to maintain this nutritional status at other stages of life, which is associated with the occurrence of cardiovascular diseases, type II diabetes, and other chronic diseases.
16–18 These findings reinforce the importance of investigating child’s nutritional status in early stages such as birth.
Studying mother’s nutritional status effects on the newborn by BMI allows lifelong follow-up through an easy-to-obtain indicator both in clinical practice and population studies. It allows conducting early interventions to prevent overweight, which currently is an enormous public health problem in several countries, with negative implications both for health and quality of life. Therefore, this study aims to evaluate the effect of prepregnancy BMI on newborns’ BMI.
MethodsThis study used data from the birth cohort “
Fatores etiológicos do nascimento pré-termo e consequências dos fatores perinatais na saúde da criança: coortes de nascimento em duas cidades brasileiras” - BRISA (Brazilian Ribeirão Preto and São Luís Birth Cohort Studies).
19 Data collection occurred in two stages: during prenatal care and at birth.
This is a convenience cohort initiated during prenatal care due to the impossibility of obtaining a random representative sample of pregnant women from the population of São Luís, state of Maranhão because it lacked reliable records of pregnant women or those who sought prenatal care.
The sample consisted of pregnant women and their newborns delivered in public and private maternity hospitals at São Luís - MA, from February 2010 to June 2011.
19During prenatal care, the pregnant women answered a questionnaire that investigated the following information: maternal age, schooling level, occupation, marital status, socioeconomic status, alcohol use and maternal smoking during pregnancy, prenatal care, presence of hypertension and diabetes at the end of pregnancy, and weight prior to pregnancy. Their height was measured witha portable stadiometer (Alturexata®) according to Lohman recommended techniques.
20The following information was used from the questionnaire applied at the delivery: gestational age, parity, and delivery method. Data from the NB questionnaire, such as gender, were also used. The baby’s birth weight and length measurements were assessed by routine hospital staff and were obtained from the NB’s chart.
The exposure variable was the prepregnancy BMI, which was used continuously and categorically. This categorical variable classified prepregnancy BMI as low birth weight (BMI<18.5kg/m
2), eutrophic (18.5kg/m
2≤BMI≤24.9kg/m
2), overweight (25.0kg/m
2≤ BMI≤29.9kg/m
2), and obesity (BMI≥30.0kg/m
2).
The variables were categorized as follows: maternal age (dummy variable with three categories: 1 - under 20 years, 2 -20 to 34 years, and 3 -35 years or more), parity (categorized considering whether the woman had 1 - just one delivery, 2 -two deliveries, 3 -three or more deliveries), maternal occupation (non-manual, specialized/semi-specialized manual, and no occupation - unemployed/student), socioeconomic status as defined by the
Associação Brasileira de Empresas de Pesquisa (Brazilian Association of Population Studies -ABEP, 2015, categorized in A/B, C, and D/E), maternal education (0 to 7 years -attended preschool and elementary school; 8 to 12 years -completed or partially completed high school; >12 years -incomplete or complete higher education), marital status (with partner -married/domestic partnership; and without partner -single/divorced/widowed), alcohol use during pregnancy (yes or no), smoking during pregnancy (yes or no) and gestational age (GA) as a continuous variable; delivery method (vaginal and cesarean section), prenatal care (yes or no), hypertension and gestational diabetes (yes or no) reported by the woman from a medical diagnose, and gestational weight gain, which was a continuous variable.
GA was estimated based on two criteria: Ultrasound (UO) performed with less than 20 weeks of GA and the date of last menstrual period (LMP). When GA measured by LMP differed for more or for less in ten days compared to the value estimated by the UO, the GA was estimated by the LMP; otherwise, it was estimated based on UO.
21The following NB data were used: gender (male or female), and weight and length at birth. The outcome was the BMI of the NB analyzed in two ways, as a continuous variable and categorized according to the WHO curves (2006), classifying the NB as: thinness (<z-score -2), eutrophic (≥z-score -2 and <z-score +2), and overweight and obesity (≥z-score +2).
Based on the literature, a theoretical model was constructed to analyze the association between prepregancy BMI and newborn BMI using the Directed Acyclic Graph (DAG), elaborated using the Dagitty browser
®. From the elaborated DAG, the Dagitty
® shows the minimum adjustment necessary to estimate the association of interest. The minimum set of variables needed to estimate the effect of prepregnancy BMI on NB’s BMI was: Maternal schooling level, maternal age, and parity.
To characterize the sample of mother-child binomial, the relative and absolute frequencies of all variables evaluated were presented.
Multiple linear regression analysis was used to identify the linear association between prepregnancy BMI and NB’s BMI (continuous variables), using the model suggested by the DAG. The Beta coefficient of linear regression (ß) and the respective confidence intervals (CI95%) were estimated. To check the assumptions of the linear regression model and identification of outliers
, residue analysis was performed for both models.
Poisson analysis with robust estimation of variance was used to estimate the relative risk (RR) and CI95% of the risk of overweight among NBs of overweight and obese mothers according to prepregnancy BMI. For adjustment, the variables suggested by the DAG were also considered.
To verify ifthe study variables followed the normal distribution pattern, distribution graphs (histograms) were constructed, and the Shapiro Wilk test was applied. For all analyses, the significance level was set at 5%.All analyses were performed on STATAprogram, version 14.0.
The research was approved by the Research Ethics Committee of the Hospital Universitárioof the Universidade Federal do Maranhão (HUUFMA)with a consubstantiated opinion under protocol n
o. 223/2009, CAAE: 4471/2008-30.
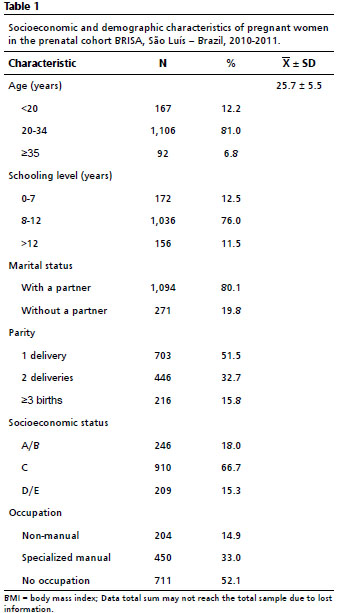
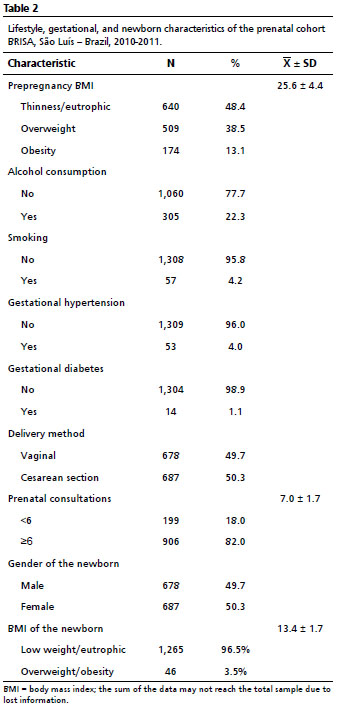
ResultsThe sample consisted of 1,365 pregnant women and their newborns. Among NBs of the São Luís cohort, 50.3% were female, and the average BMI at birth was 13.4±1.7 kg/m
2. The prevalence of overweight among NBs was 3.5%. In the sample, 81.0% of the mothers were in the age group of 20 to 34 years, 76.0% had 8 to 12 years of study, 66.7% were in socioeconomic status C, 52.1% were without occupation, and 80.1% lived with a partner (Table 1). Cesarean section was performed by 50.3%, and 82.0% had more than six prenatal consultations. Regarding prepregnancy BMI, 51.6% started pregnancy overweight (Table 2).
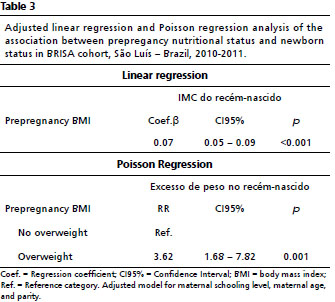
Prepregnancy BMI was linearly associated with newborn’s BMI (Coef). ß= 0.07; CI95%= 0.05-0.09;
p<0.001) (Table 2). The increase of 1kg/m
2 in prepregnancy BMI increased newborn’s BMI by 0.07kg/m
2. Children of mothers who were overweight in the prepregnancy period had a higher risk of being overweight at birth (RR=3.58; CI95%= 1.66–7.74;
p<0.001) (Table 3).
DiscussionIn this study, the increase in prepregnancy BMI was associated with higher BMI values of the newborn linearly and independently. Starting pregnancy while overweight was a risk factor for overweight innewborns.
A self-reported prepregnancy weight may be underestimated or subjected to memory bias information, thus representing a limitation of the study. Nevertheless, using self-reported weight in population studies with pregnant women is common due to the high occurrence of unplanned pregnancy. Moreover, according to Schmidt,
22 this measure is considered sufficiently validated to use in studies on the prevalence of obesity. Furthermore, Fonseca
et al.23 concluded that self-reported and measured weight and height information showed good agreement and validity, reinforcing the possibility of using self-reported data.
To this date, we found no studies that evaluated the relation between prepregnancy BMI and newborns’ BMI in Brazil. In Lisbon, Portugal, a study with 100 mother-baby binomials from a convenience hospital sample identified that maternal pregestational BMI was associated with higher newborn BMI measured within 72 hours after delivery (ß=1.16; CI95%=0.49–1.83).
24 Evidence shows that pregestational overweight contributes to higher birth weight of newborns and increased risk of being born large for gestational age (LGA).
7–9 Women who begin their pregnancy overweight/obese may expose the fetus to a “hypernutrition,” creating an intrauterine environment with excess glucose and fat, stimulating insulin resistance and hyperglycemia, leading to accelerated fetal growth.
5,25A meta-analysis conducted by Voerman
et al.15 found that a higher prepregnancy BMI was associated with increased risk of overweight/obesity in children and adolescents aged from 2 to 18 years, with stronger effects observed as age increased. This study showed that the effect of prepregnancy BMI on the child’s BMI is already observed since birth, pointing to the earliness of this effect in transferring excess weight between generations.
The association between prepregnancy BMI and its effects on the nutritional status of children have been reported not only by using BMI as an indicator, but also by measuring adiposity, which suggests a direct relation between prepregnancy BMI and fat mass at birth. Starling
et al.26 found that newborns up to three days old born to mothers who were overweight or obese had a higher percentage of fat and fat mass compared to those born to mothers with normal BMI. Carlsen
et al.27also found that newborns of obese mothers had higher fat mass and abdominal fat accumulation. These results are very relevant; however, obtaining measures of adiposity in such early stages is complex not only due to the age of newborns, but also because the equipment that measures body composition is more expensive than those for BMI calculation. All these aspect may difficult the use of adiposity indicators in the routine follow-up.
The literature has recommended using BMI in children under two years because it measure the proportionality between weight and height, with a high correlation with the weight-for-height indicator, which is highly used to assess the nutritional status of children in this age group.
10 Moreover, BMI has the advantage of being an index capable of predicting the risk of obesity and other diseases in childhood and at other stages of life.
11,16,18,28 Furthermore, BMI can be used throughout life since birth, and does not require the transition of indicators for nutritional assessment during childhood.
10,11We should also highlight that variables such as alcohol consumption and smoking were evaluated only qualitatively, disregarding quantitative aspects that may increase the risk in groups with higher alcohol and tobacco consumption. Moreover, we lack information on maternal food intake, which could also interfere with the studied association.
Due to the lack of reliable records of pregnant women in São Luís - MA, a convenience sample was used, which may compromise the generalization of these results. Since the data were collected in 2010, they may not represent the current situation anymore. However, they showed an association between prepregnancy BMI and newborns’ BMI during the study period.
As strengths, we highlight the large sample size and the cohort design, which allowed us to study the cause and effect relation with high statistical power. Furthermore, the selection of variables by means of the DAG-based causal theoretical model for evaluating the association between prepregnancy BMI and newborns’BMI, allowed for effect estimation, minimizing the occurrence of confounding and selection biases. To the best our knowledge, few studies have evaluated children’s BMI at such an early stage of life.
This study shows that the newborns’BMI was able to indicate the risk of prepregnancy overweight on the nutritional status of the child. Therefore, our results show the importance of preventing overweight from the prepregnancy period, since it has a greater influence on the nutritional status of the child than weight gain during pregnancy.
15 Thus, besides controlling weight gain during pregnancy, women who wish to conceive should consider nutritional planning to maintain or achieve a healthy weight.
Moreover, health professionals should use BMI to assess nutritional status from birth, as it is an indicator that allows for lifelong monitoring and predicts the risk of chronic noncommunicable diseases in childhood and later in life.
AcknowledgmentsWe thank the financial support:
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq),
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP),
Fundação de Amparo à Pesquisa do Estado do Maranhão (FAPEMA),
Programa de Apoio aos Núcleos de Excelência (PRONEX),
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References1. Zhang P, Wu J, Xun N. Role of maternal nutrition in the health outcomes of mothers and their children: A retrospective analysis. Med Sci Monit. 2019; 25: 4430-7.
2. World Health Organization (WHO). Exclusive breastfeeding; Complementary feeding; Vitamin A.
In: Essential Nutrition Actions: improving maternal,newborn, infant and young child health and nutrition. Geneva: WHO; 2013. [access in 2020 Jul 13]. Available from:
https://apps.who.int/iris/bitstream/handle/10665/84409/9789241505550_eng.pdf;jsessionid=CE294D54A5D48FB6614DDBFF2660169B?sequence=13. Woldeamanuel GG, Geta TG, Mohammed TP, Shuba MB, Bafa TA. Effect of nutritional status of pregnant women on birth weight of newborns at Butajira Referral Hospital, Butajira, Ethiopia. SAGE Open Med. 2019; 7: 2050312119827096.
4. Wierzejska R, Wojda B. Pre-pregnancy nutritional status versus maternal weight gain and neonatal size. Rocz Panstw Zakl Hig. 2019; 70 (4): 377-84.
5. Nehab SRG, Villela LD, Abranches AD, Rocha DM, Silva LML, Amaral YNV,
et al. Influence of gestational and perinatal factors on body composition of full-term newborns. J Pediatr (Rio J). 2020; 96(6); 771-7.
6. McCloskey K, Ponsonby AL, Collier F, Allen K, Tang MLK, Carlin JB,
et al. The association between higher maternal pre-pregnancy body mass index and increased birth weight, adiposity and inflammation in the newborn. Pediatr Obes. 2018; 13 (1): 46-53.
7. Alberico S, Montico M, Barresi V, Monasta L, Businelli C, Soini V,
et al. Multicentre Study Group on Mode of Delivery in Friuli Venezia Giulia. The role of gestational diabetes, pre-pregnancy body mass index and gestational weight gain on the risk of newborn macrosomia: Results from a prospective multicentre study. BMC Pregnancy Childbirth. 2014; 14 (23): 1-8.
8. Gao X, Yan Y, Xiang S, Zeng G, Liu S, Sha T,
et al. The mutual effect of pre-pregnancy body mass index, waist circumference and gestational weight gain on obesity-related adverse pregnancy outcomes: A birth cohort study. PLoS One. 2017; 12 (6): 1-13.
9. Nowak M, Kalwa M, Oleksy P, Marszalek K, Radon-Pokracka M, Huras H. The relationship between pre-pregnancy BMI, gestational weight gain and neonatal birth weight: A retrospective cohort study. Ginekol Pol. 2019; 90 (1): 50-4.
10. Furlong KR, Anderson LN, Kang H, Lebovic G, Parkin PC, Maguire JL,
et al. BMI-for-Age and weight-for-length in children 0 to 2 years. Pediatrics. 2016; 138 (1): 1-9.
11. Roy SM, Spivack JG, Faith MS, Chesi A, Mitchell JA, Kelly A,
et al. Infant BMI or Weight-for-Length and Obesity Risk in Early Childhood. Pediatrics. 2016; 137 (5): e20153492.
12. Marchand V. Author’s answer: Promoting optimal monitoring of child growth in Canada: Using the new World Health Organization growth charts – Executive Summary [Letter]. Paediatr Child Health. 2010; 15 (5): 258.
13. Hall DMB, Cole TJ. What use is the BMI? Arch Dis Child. 2006; 91 (4): 283-6.
14. Daniels SR. The Use of BMI in the Clinical Setting. Pediatrics. 2009 Sep; 124 (Supl. 1): S35-41.
15. Voerman E, Santos S, Golab BP, Amiano P, Ballester F, Barros H,
et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med. 2019; 16 (2): 1-22.
16. Charakida M, Deanfield JE. BMI trajectories from childhood: the slippery slope to adult obesity and cardiovascular disease. Eur Heart J. 2018; 39: 2271-3.
17. Buscot M-J, Thomson RJ, Juonala M, Sabin MA, Burgner DP, Lehtimaki T,
et al. Distinct child-to-adult body mass index trajectories are associated with different levels of adult cardiometabolic risk. Eur Heart J. 2018; 39: 2263-70.
18. Péneau S, Giudici KV, Gusto G, Goxe D, Lantieri O, Hercberg S,
et al. Growth Trajectories of Body Mass Index during Childhood: Associated Factors and Health Outcome at Adulthood. J Pediatr (Rio J.). 2017; 186: 64-71.
19. Silva AAM, Simões VMF, Barbieri MA, Cardoso VC, Alves CMC, Thomaz EBAF,
et al. A protocol to identify non-classical risk factors for preterm births: The Brazilian Ribeirão Preto and São Luís prenatal cohort (Brisa). Reprod Health. 2014; 11 (79): 1-9.
20. Lohman T, Roche A, Martorell R. Anthropometric Standardization Reference Manual. Chicago: Human Kinetics Books; 1988.
21. Verburg BO, Steegers EAP, De Ridder M, Snijders RJM, Smith E, Hofman A,
et al. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population-based cohort study. Ultrasound Obstet Gynecol. 2008; 31 (4): 388-96.
22. Schmidt MI, Duncan BB, Tavares M, Polanczyk CA, Pellanda L, Zimmer PM. Validity of self-reported weight--a study of urban Brazilian adults. Rev Saúde Pública. 1993; 27 (4): 271-6.
23. Fonseca MJM, Faerstein E, Chor D, Lopes CS. Validity of self-reported weight and height and the body mass index within the “Pró-saúde” study. Rev Saúde Pública. 2004 Jun; 38 (3): 392-8.
24. Pereira-Da-Silva L, Cabo C, Moreira AC, Virella D, Guerra T, Camoes T,
et al. The Adjusted Effect of Maternal Body Mass Index, Energy and Macronutrient Intakes during Pregnancy, and Gestational Weight Gain on Body Composition of Full-Term Neonates. Am J Perinatol. 2014; 31 (10): 875-81.
25. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestationaldiabetes mellitus. Obstet Gynecol. 2010; 115 (3): 597-604.
26. Starling AP, Brinton JT, Glueck DH, Shapiro AL, Harrod CS, Lynch AM,
et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr. 2015, 101 (2): 302-9.
27. Carlsen EM, Renault KM, Nørgaard K, Nilas L, Jensen JEB, Hyldstrup L. Newborn regional body composition is influenced by maternal obesity, gestational weight gain and the birthweight standard score. Acta Paediatr. 2014; 103 (9): 939-45.
28. Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med. 2017; 377 (22): 2145-53.
Received on March 18, 2021
Final version presented on May 17, 2022
Approved on 27 August, 2022
Authors’ contribution: Araújo AP analyzed and interpreted the data, and elaborated the first version of the manuscript. Carvalho CA and Viola PCAF analyzed and interpreted the data, and wrote and reviewed the manuscript. Ribeiro CCC and Barbosa JMA interpreted the data and reviewed the manuscript. Simões VF concepted the article proposal, conducted the project, interpreted the data, wrote, and reviewed the manuscript. The authors approved the final version of the article and declare no conflict of interest.
 ; Carolina Abreu de Carvalho2
; Carolina Abreu de Carvalho2 ; Cecilia Claudia Costa Ribeiro 3
; Cecilia Claudia Costa Ribeiro 3 ; Janaina Maiana Abreu Barbosa 4
; Janaina Maiana Abreu Barbosa 4 ; Poliana Cristina de Almeida Fonseca Viola 5
; Poliana Cristina de Almeida Fonseca Viola 5 ; Vanda Maria Ferreira Simões 6
; Vanda Maria Ferreira Simões 6