ABSTRACT
OBJECTIVES: to evaluate the evolution of extremely preterm and very preterm infants admitted to neonatal intensive care units, regarding the use of ventilatory support, morbidities, medication use, death, survival and viability.
METHODS: a non-concurrent cohort study, with 163 very premature and extreme newborns hospitalized in three neonatal intensive care units, during 2016 and 2017. A descriptive analysis of the data obtained from the medical records was performed. The outcomes studied were the use of ventilatory support, morbidities, medication use, death and causes of death. A survival curve was constructed and a viability limit was defined.
RESULTS: in the study, 28.2% were extreme and 71.8% were very premature. In this order of subgroups, the need for mechanical ventilation was higher for the extremes (65.2% and 41.0%) and the main diagnosis was early sepsis (78.6% and 82.6). Off-label (60.5% and 47.9%) and off-license (25.3% and 29.0%) medications were used. Most deaths (57.8%) occurred between the extremes, mainly due to septic shock. Survival was lower for the lowest gestational ages and the limit of viability was between 26 and 27 weeks.
CONCLUSIONS: the main morbidities were from the respiratory system, with high use of off-label and unlicensed medications. Extremes had a greater demand for intensive care in addition to needing more drugs and progressing more to death.
Keywords:
Pharmacoepidemiology, Extremely premature infant, Critical care, Intensive care units, neonatal
RESUMO
OBJETIVOS: avaliar a evolução dos prematuros extremos e muito prematuros internados em unidades de terapia intensiva neonatais, quanto ao uso de suporte ventilatório e de medicamentos, óbito, sobrevida e viabilidade.
MÉTODOS: estudo de coorte não concorrente, com 163 recém-nascidos muito prematuros e extremos internados em três unidades de terapia intensiva neonatais, durante 2016 e 2017. Realizou-se análise descritiva dos dados obtidos dos prontuários. Os desfechos estudados foram o uso de suporte ventilatório, morbidades, uso de medicamentos, óbito e causas de óbito. Foi construída curva de sobrevivência e delimitado um limite de viabilidade.
RESULTADOS: no estudo, 28,2% eram extremos e 71,8% muito prematuros. Nessa ordem de subgrupos, a necessidade de ventilação mecânica foi maior para os extremos (65,2% e 41,0%) e o principal diagnóstico foi sepse precoce (78,6% e 82,6).Medicamentos off-label (60,5% e 47,9%) e sem-licença (25,3% e 29,0%) foramutilizados. A maioria dos óbitos (57,8%) ocorreu entre os extremos, principalmente por choque séptico. A sobrevivência foi menor para as menores idades gestacionais e o limite de viabilidade ficou entre 26 e 27 semanas.
CONCLUSÕES: as principais morbidades foram do sistema respiratório, com alto uso de medicamentos off-label e sem licença. Extremos tiveram maior demanda de cuidados intensivos além de necessitarem de mais medicamentos e evoluírem mais ao óbito.
Palavras-chave:
Farmacoloepidemiologia, Recém-nascido prematuro, Cuidados críticos, Unidades de terapia intensiva neonatal
IntroductionPrematurity is globally the main cause of death in children under five years of age. The same occurs in Brazil, with a concentration of over 60% of infant deaths in the neonatal period.
1The World Health Organization (WHO) defines preterm birth as that birth which occurs before 37 weeks of pregnancy.
2Despite preterm newborns generally have typical complications of this population, some groups are more vulnerable than others. In a meta-analysis carried out in high-income countries, in the period from 200 to 2017, it was observed a high variation in the survival rate, which was low for the gestational age (GA) under 25 weeks, close to 25%. After 27 weeks, the survival rate increased to 90%, which demonstrates that survival increases with the GA.
3 In this context, it is understood that many preterm infants (28 weeks and less than 32 weeks) and extreme preterm infants (less than 28 weeks) are especially vulnerable due to the physiological immaturity that is notably increased in these groups.
4Furthermore, extreme prematurity also indicates higher association with neonatal complications and worse clinical outcomes compared to moderate preterm infants (32 weeks to less than 34) and late preterm infants (34 weeks to less than 37), which is reflected in high premature mortality rates, as well as hospitalizations, surgeries and use of medication.
5 The diverse associated morbidities, in addition to interfere in the response to intensive care, lead the pharmacological treatment for this population to be challenging, since most of medications routinely used have their prescriptions based on results of surveys focused on adults, which differ from children in several aspects, including the pharmacological response.
6 Given the above, drug treatment for this population is mostly empirical, characterized by the recurrent usage of off-label and unlicensed drugs. It is considered as off-label drugs those that target age, indication and way of administration diverge from what is authorized by the competent health agency, in this case the Food and Drug Administration - FDA. On the other hand, medications without registry, those contraindicated for neonatology (did not present safety or effectiveness), as well as compounding pharmacy preparations through medical prescription, or modified by professionals without FDA regulation,
7 were classified as unlicensed.
All of these characteristics indicate that the components of intensive care during preterm hospitalization are determinative for the outcome of these patients. This study aims to describe the demand for intensive care, the main morbidities, the drug use, the causes of that and the viability between extreme preterm infants and very preterm infants, besides building information that may contribute with the clinical practice based on evidence.
MethodsA cohort study, hospital based, part of the research "Premature Birth Cohort - Survival and morbidity in premature infants in Neonatal Intensive Care Units (NICU) in the municipality of Vitória da Conquista - BA: a non-concurrent cohort study". The studied hospital units served as an internship field for the medical residency program in pediatrics and neonatology, and possessed protocol for similar clinical practices.
All extreme premature and very premature infants hospitalized in NICUs were included in the study, from January 1, 2016 to December 21, 2017. The population was studied from the day of admission until 27 days of life.
The sample for the original study was obtained by convenience (n=400). However, the smaller sample size necessary to represent the premature infant population in the region was estimated in 384, considering the following parameters: infinite population size (datum in which it is not possible to estimate the total of preterm infants that would need neonatal intensive care), expected frequency of 50% (considering the multiple outcomes assessed), 5% precision and confidence interval of 95%. For the analysis of this article, a cutoff was made, using as sample preterm infants with GA between 22 and less than 32 weeks. Finally, 163 preterm infants remained in this study.
Data were collected by means of the analysis in the preterm infants' medical records, stored at the service of medical files and statistics of hospitals. The following major congenital abnormalities were used as exclusion criteria: complex congenital heart diseases, gastrointestinal tract atresia, abdominal wall defect, hydrocephaly, encephalocele and diaphragmatic hernia.
The tool used to perform the collection was an adapted questionnaire from the National Survey
Nascer no Brasil (Born in Brazil).
8 Volunteer healthcare researchers under supervision of neonatologists were responsible for data collection, using a digital questionnaire with the use of tablets with Koobo Toolbox 1.4.8 software. Data collection of the main field occurred between June 2018 and May 2019.
The dependent variable was the evolution of preterm infants. The considered outcomes were the demand for intensive care, main morbidities and the use of medication. It was also assessed the occurrence of deaths in this population, and the period in which they occurred. The survival curves and viability curves for the evaluated groups were also calculated.
In order to obtain GA, it was preferably used the date of the last menstruation, followed by early ultrasound. In face of the impossibility of these findings, the assessment of physical and neurological signs of newborns was used, by means of the Capurro of New Ballard scales.
The analyzed variables were: sex (male; female), birth weight (low weight; very low weight and extreme low weight), Apgar test after the fifth minute (≥ 7; < 7), type of delivery (vaginal; cesarean), use of surfactant in the birth room (no;yes), use of surfactant in NICU (no;yes), cardiopulmonary resuscitation in the birth room (no; only ventilation with positive pressure ventilation (PPV); advanced resuscitation: positive pressure ventilation with cardiac massage and/or use of medication), time of mechanical ventilation during hospitalization (0 day; 1 to 5 days, six days or more), city of origin of the mother (Vitória da Conquista; another city) and place of birth and hospitalization (same hospital; different hospitals, in the same city; birth in another city or in transit). The neonates with z score of birth weight under -1.29 (percentile 10%), defined in agreement with the Intergrowth-21 curves, were considered small for gestational age, categorized in yes or no.
9The main diseases developed in the period of 27 days were also described, according to the preterm subgroups: apnea, early respiratory distress, necrotizing enterocolitis, neonatal jaundice, early sepsis, late sepsis and respiratory distress syndrome (RDS) or hyaline membrane disease (HMD).
The use of medications was obtained for the period of hospitalization in NICU, according to the GA subgroup. Each pharmaceutical specialty was registered under the generic name, pharmaceutical form and way of administration. Besides the specialties (drugs) all the daily doses prescriptions were registered, obtained as number of analysis the number of total prescriptions. The pharmacological classification was performed according to the Anatomical Therapeutic Chemical (ATC) classification, preconized by the WHO.
2 For the present study, we used the classifications of drugs concerning level 1 (anatomical) and level 2 (therapeutic). The drugs were also classified as off-label and unlicensed for the population according to Costa
et al. ,
7 with the use of the international database Drug Dex-Micromedex.
10The occurrence of death for extreme and very preterm infants, during the neonatal period (first 27 days of life) was described and categorized in yes and no. Furthermore, the deaths were distributed according to the time of occurrence after birth (early neonatal period if occurred within 6 days of life or less, and late neonatal if occurred from 7 to 27 days). The main death causes were also described: septic shock, multiple organ failure, respiratory distress syndrome (or hyaline membrane disease), acute renal failure and pulmonary hemorrhage.
In this research, the time of follow-up onset was the date of birth of each patient and the time of follow-up was until de 27th day of life or the occurrence of death. Since the follow-up was daily, the standard was
½ of the period for deaths occurring within less than 24 hours of life. In this way, patients were followed for different times and death was the censoring event. The survival curve was performed to demonstrate the time of survival, during follow-up, of the subgroups of GA under 32 weeks: 23 to 15 weeks, 26 to 27 weeks, 28 to 29 weeks and 30 to 31 weeks and the Log Rank test was calculated between these groups and neonatal death. The evaluation of viability limit corresponds to gestational age in which the newborn presents 50% or more of survival chance outside the womb, and for this evaluation, the previously described subgroups were also used.
11First, the descriptive analysis by means of absolute and relative frequencies was performed. In order to describe diseases in this population at the neonatal period, the incidence of each of them was calculated. For all these mentioned variables, the differences between the two gestational age groups were compared with the Pearson's chi square test or Fisher's exact test. Concerning the evaluation of drug use (total, off-label and unlicensed), descriptive data analysis were carried out by means of simple frequency distribution, using the total of dose prescriptions as an analysis unit. For the construction of the survival curve, the Kaplan-Meier nonparametric method was used, and, when presenting viability, a bar graph with the subgroups of GA under 32 weeks was created. The Stata version 15.0 software (Stata Corporation, College Station, USA) was used in data analysis.
The research was approved by the Research and Ethics Committee of the Multidisciplinary Health Institute of the Federal University of Bahia (CAAE:79450717.4.0000.5556).
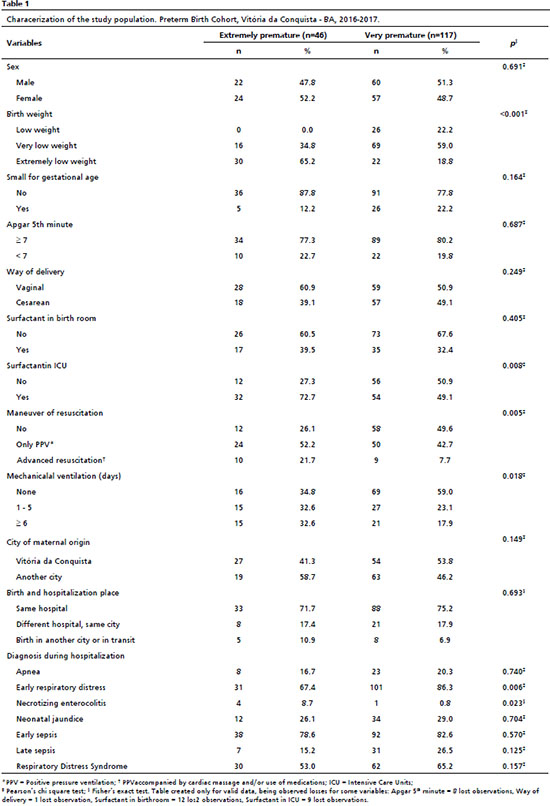
ResultsOf 163 preterm infants, gestational age varied from 23 weeks to less than 32 weeks, with 46 (28.2%) of extreme preterm and 117 (71.8%) very preterm, with a statistically significant difference between the groups. Among extreme premature infants, over half were of female sex (52.2%) and had extreme low birth weight (65.2%). The classification as low for gestational age in this subgroup was observed in 12.2%; the Apgar score of fifth minute was <7 in 22.7%, and 60.9% were born via vaginal delivery. It was observed the use of surfactant in birth room (39.5%), although it was more frequent in NICU (72.7%). Maneuver of resuscitation only with PPV was used in 52.2%, while 21.7% needed advanced resuscitation in the birth room. For the last two variables, a significant difference was observed between the groups. The percentile of patients that needed or not mechanical ventilation was almost equivalent between those who did not use it (34.8%), those who used for 1 to 5 days (32.6%) and those who used for a period equal or higher than 6 days (32.6%) (Table 1).
 ; Joice Silva Machado2
; Joice Silva Machado2 ; Daiane Borges Queiroz3
; Daiane Borges Queiroz3 ; Renart Santos Costa4
; Renart Santos Costa4 ; Verônica Cheles Vieira5
; Verônica Cheles Vieira5 ;Raquel Cristina Gomes Lima6
;Raquel Cristina Gomes Lima6 ; Danielle Souto de Medeiros7
; Danielle Souto de Medeiros7