ABSTRACT
OBJECTIVES: to assess the prevalence and epidemiological factors associated with group B Streptococcus (GBS) colonization in pregnant women in Porto Velho City, Rondônia.
METHODS: GBS was identified and isolated by genotypic and microbiological methods from rectovaginal samples of pregnant women between 35 and 37 weeks of gestation. Epidemiological data were collected using questionnaires and their correlation with colonization was assessed. The antimicrobial susceptibility profile was determined by disk diffusion method.
RESULTS: a total of 22.5% (102/453) pregnant women were colonized with GBS. A higher level of colonization was observed at the vaginal tract (17.6%), compared to the rectal area. We did not find any sociodemographic or obstetric factors associated with an increased risk of GBS colonization. All strains were susceptible to antibiotics penicillin, ampicillin, cefazolin, and ceftriaxone. In contrast, the rates of resistance to tetracycline (74.1%), erythromycin (14.1%), and clindamycin (3.5%) were observed.
CONCLUSION: the prevalence of GBS as well as the absence of predictors of colonization demonstrated the need for universal screening for GBS in all pregnant women in the region. In addition, we showed that the first-line antibiotics recommended for prophylaxis are still good options for the prevention of neonatal GBS disease in the region.
Keywords:
Antibiotic resistance, Neonatal diseases, Antibiotic prophylaxis, Public health surveillance, Pregnant women
RESUMO
OBJETIVOS: avaliar a prevalência e os fatores epidemiológicos associados à colonização por Streptococcus do grupo B (GBS) em gestantes na cidade de Porto Velho, Rondônia.
MÉTODOS: GBS foi identificado e isolado por métodos genotípicos e microbiológicos a partir de amostras retovaginais de grávidas com 35-37 semanas de gestação. Os dados epidemiológicos foram coletados através de questionários e sua correlação com a presença de colonização foi avaliada. O perfil de susceptibilidade antimicrobiana foi determinado pelo método de disco-difusão.
RESULTADO: um total de 22.5% (102/453) gestantes foram colonizadas por GBS. Um nível mais alto de colonização foi observado no sítio vaginal (17.6%) em comparação ao sítio retal. Não encontramos nenhum fator sociodemográfico ou obstétrico associado a um risco aumentado de colonização por GBS. Todas as amostras foram suscetíveis aos antibióticos penicilina, ampicilina, cefazolina e ceftriaxona. Em contraste, as taxas de resistência à tetraciclina (74.1%), eritromicina (14.1%) e clindamicina (3.5%) foram observadas.
CONCLUSÕES: a prevalência de GBS, bem como a ausência de preditores de colonização, demonstraram a necessidade de triagem universal para GBS em todas as gestantes da região. Além disso, mostramos que os antimicrobianos de primeira linha recomendados para profilaxia são boas opções para a prevenção da doença GBS neonatal na região.
Palavras-chave:
Resistência a antibióticos, Doenças neonatais, Profilaxia por antibióticos, Vigilância em saúde, Gestantes
IntroductionGroup B
Streptococcus or
Streptococcus agalactiae (GBS) is the major etiological agent of neonatal infections.
1 GBS mainly colonizes the genitourinary and gastrointestinal tracts of humans; however, colonization in the vaginal and rectal areas of pregnant women poses a risk to the health of newborns because the GBS can be vertically transmitted before, during, and after childbirth. Consequently, GBS can cause a wide range of newborn clinical diseases such as sepsis, meningitis, and pneumonia.
1The pathology of GBS can be classified according to the time at which symptoms of the disease first appear. Early onset disease describes the appearance of symptoms within the first seven days of the newborn's life and results from spread of GBS through the ascending pathway of the uterus or at delivery. Severe infections often present as sepsis, pneumonia, cardiovascular instability, or, less frequently, meningitis, and are characterized by adverse clinical evolution.
2 Late onset disease is characterized by symptom onset between the eighth day of life to three months and can also be associated with microorganisms other than GBS, including coagulase-negative staphylococci,
Escherichia coli, and other gram-negative bacteria
2. Late onset disease is mainly associated with meningitis, which can lead to cognitive and neurological sequelae.
3 Invasive diseases caused by GBS account for 5–20% of mortality in premature newborns and 1–8.4% of mortality in full-term newborns.
4Epidemiological investigations have shown that approximately 18% of women worldwide are colonized by GBS during pregnancy, though the incidence varies between 11% and 35% according to region
1. GBS is associated with 518,000 preterm births, 392,000 neonatal infections, and 91,000 neonatal deaths worldwide annually.
5 Studies on the prevalence of colonization in pregnant women in Brazil have shown heterogeneous rates of 4.2–28.4%.
6 Concerningly, low-income countries are more susceptible to high morbidity and mortality rates due to GBS than high-income countries, which is associated with a lack of systematic prophylaxis measures, among other factors.
7The guidelines for GBS prophylaxis were updated in 2020 by the American College of Obstetricians and Gynecologists. Universal GBS screening involves the screening of maternal colonization through rectal and vaginal cultures collected at 36-37 weeks of gestation. Positive results are then managed with intrapartum antimicrobial prophylaxis (IAP). Penicillin G is the first-choice antibiotic for the treatment of such cases. Alternative drugs, such as clindamycin and cefazolin, are used in pregnant women allergic to penicillin. The majority of GBS isolates remain susceptible to penicillin and other β-lactams; however, resistance to antimicrobial agents used as alternative therapies, primarily lincosamides, has been described previously.
8In Brazil, GBS has not been acknowledged as the causative agent of underlying infectious processes affecting newborns and pregnant women owing to failure to isolate the microbe and/or underreporting. This lack of acknowledgement occurs despite the severity of GBS infection and the fact that the population is highly likely to benefit from prophylaxis. In Brazil, there is no recommendation by national health authorities for GBS screening of low-risk pregnant women, which is justified by a shortage of national research substantiating the development of guidelines recommending care provision to GBS carriers.
9In the Brazilian Amazon, particularly in the state of Rondônia, there is a lack of epidemiological data on multiple important infectious diseases, including GBS, which is in contrast to the Southeast of Brazil.
6 The Brazilian Amazon covers over 6.3 million km
2 and has a population of around 29.6 million people, and there is substantial variation in many lifestyle factors across the region.
10 There is a pressing need for medical research on topics such as bacteriology in pregnant women in the Amazon. Such research could help to improve medical and social policies affecting both mother and child and thus drive a decrease in child mortality in the Amazon. Given the clinical importance of this pathogenic agent and the lack of research on GBS prevalence in Northern Brazil, this study aimed to assess the prevalence of GBS colonization, and the sociodemographic and clinical characteristics associated with pregnant women attending the Public Healthcare Network in the city of Porto Velho, state of Rondônia, Brazil.
MethodsA prospective, cross-sectional study was conducted from April 2018 to March 2020 in the city of Porto Velho, the capital of the state of Rondônia, which is located in the western part of the Northern Region of Brazil, in the Western Amazon, and has an estimated population of 548.952 people.
The study population comprises pregnant women randomly sampled between 35 to 37 weeks of gestation, according to the 2010 version of the CDC's perinatal GBS guidelines,
11 who were assessed at the Mother and Child Integrative Center, as well as low-risk pregnant women assessed at eleven Basic Healthcare Units in the city of Porto Velho. Pregnant women undergoing treatment with oral or intravaginal antibiotics or at a late delivery stage were excluded from the study. Based on a 20% prevalence of GBS in women and using a sampling error of 5% and confidence level of 95%, we calculated the optimal sample size as 477 subjects.
Rectal and vaginal secretion samples were collected by healthcare professionals during routine prenatal outpatient visits. Vaginal samples were collected with a sterile swab, which was introduced into the vagina up to its distal third without previous sanitation or use of a speculum. After smooth rotation, each swab was removed and immediately immersed in the Stuart transport medium (CRAL, São Paulo, Brazil). For rectal samples, a sterile swab was introduced into the anal orifice up to the distal wall of the rectum and a smooth rotation movement was used. The swab was then immediately immersed in the transport medium (CRAL). Subsequently, samples were sent to the Microbiology Laboratory of the Oswaldo Cruz Foundation Rondônia and processed within 24 h of collection, as recommended by the Center for Disease Control and Prevention (CDC).
11 Furthermore, data on the age, civil status, ethnicity group, education level, occupation, obstetric history, and sexual activity of each patient, among other variables, were collected through questionnaires.
Swabs were removed from the transport medium and inoculated in tubes filled with 4 mL Todd-Hewitt broth (THB; KASVI, Paraná, Brazil) supplemented with gentamicin (8 µg/mL; Interlab, São Paulo, Brazil) and nalidixic acid (15 µg/mL; Sigma-Aldrich, Missouri, USA). Subsequently, the samples were incubated in 5% CO
2 at 37°C for 24 h. The bacterial DNA was extracted from the culture using the phenol–chloroform method.
12 A further 0.09 mL of broth was transferred to 2 mL cryotubes with 0.06 mL of glycerol (50%; Thermo Fisher Scientific, Massachusetts, USA) and stored in a freezer at -80°C for further bacteriological tests. The in-house GBS PCR was performed according to the method described by Ke
et al.
13 using
S. agalactiae ATCC 27956 as a positive control.
Cryopreserved samples were inoculated in THB broth and incubated in 5% CO
2 at 37°C for 24 h. Cultures were then streaked onto Columbia Blood Agar plates (BIOLOG, California, USA) supplemented with 5% defibrinated sheep blood (EBE FARMA, Rio de Janeiro, Brazil) and incubated under the aforementioned conditions.
11 Plates were assessed after 24h to check for the presence of colonies indicative of GBS, namely small (0.5–1 mm) and transparent colonies with discrete beta hemolysis. Colonies presenting these morphological and hemolytic features were subjected to presumptive identification tests for CAMP (
Christie-Atkins-Munch-Peterson) production, catalase activity, and Gram staining. After the isolation of bacteria, samples were stored in cryotubes and cryopreserved in glycerol at -80°C.
2The isolates were tested for susceptibility to the following antimicrobial agents: penicillin G (10µg), ampicillin (10µg), clindamycin (2µg), cefazolin (30µg), ceftriaxone (30µg), erythromycin (15µg), and tetracycline (30µg) (CECON, São Paulo, Brazil) using the Kirby-Bauer disk diffusion method, as recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST). The results were interpreted according to BrCast/EUCAST.
14The information from the questionnaires was collated using Excel 2016 (Microsoft, Washington, USA). Descriptive statistics were used to summarize the participants' data. Data were organized in a contingency table and analyzed using Fisher's exact test. P values ≤ 0.05 were considered significant, and 95% confidence intervals were considered.
The study was approved by the Research and Ethics Committee of the Research Center in Tropical Medicine CEP/CEPEM n. 76.812-329.
ResultsFrom April 2018 to March of 2020, 453 pregnant women were screened for GBS colonization based on eligibility criteria. The presence of GBS colonization was identified in at least one of the samples (vaginal and/or rectal) in 102 cases, giving a prevalence of 22.5% (CI95%= 19%–26%). No significant fluctuations were observed in colonization rates over the study period (Figure 1A). However, when colonization rate was stratified by the area of collection, we observed a marked growth in the rate of vaginal colonization and a slight decrease in rectal colonization over the study period (Figure 1B).
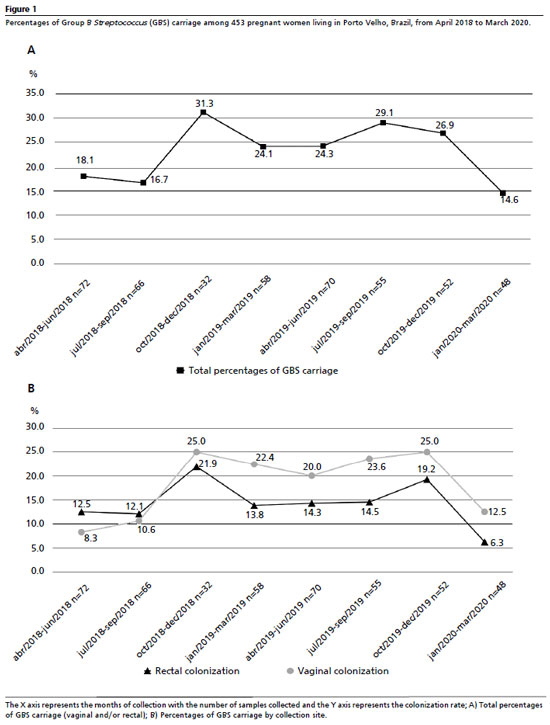
Table 1 describes the sociodemographic features based on the presence or absence of GBS colonization. Of the 453 screened pregnant women, 54.3% (246/453) were 20–30 years old, 71.7% (325/453) were married, 54.5% (247/453) were housewives, 61.4% (278/453) completed high school, 71.1% (322/453) were self-declared ‘brown', and 92.3% (418/453) lived in the urban zone. Rates of colonization by GBS in these pregnant women were as follows: aged 20–30 years (47.1%), married (71.6%), self-declared ‘brown' (73.5%), completed high school (62.7%), housewives (47.1%), and living in urban zones (93.1%). However, there were no significant differences in the presence or absence of GBS colonization in patients based on demographic characteristics. Pregnant women younger than 20 years did show a higher tendency for GBS colonization than women in other age groups (odds ratio = 1.648 [CI= 0.9661–2.813]).
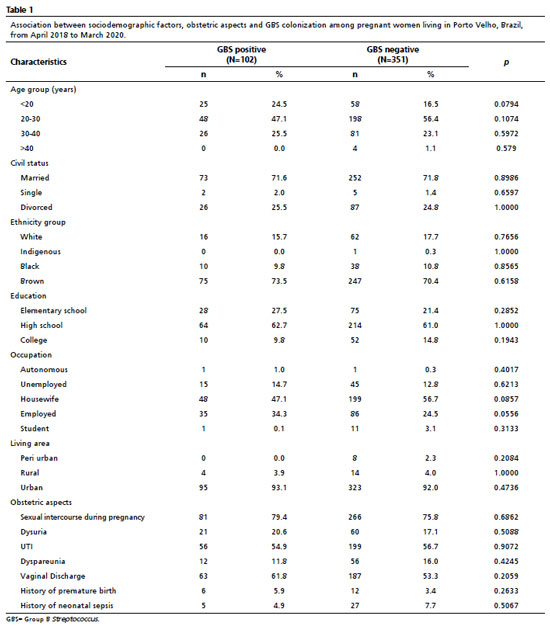
The obstetric characteristics assessed in this study are presented in Table 1. In total, 79.4% (81/102) of pregnant women who tested positive for GBS colonization had sexual intercourse during pregnancy, with a reasonable rate of urinary tract infections of 54.9% (56/102) and vaginal discharge of 61.8% (63/102). It was also observed that 5.9% (6/102) of these women had preterm delivery in a previous pregnancy, and 4.9% (5/102) reported previous neonatal sepsis. There was no significant difference between the clinical-obstetric variables and GBS colonization. Systemic arterial hypertension was the most prevalent pre-existing syndrome among the investigated patients.
The vaginal area had the highest GBS colonization rate, of 17.6% (80/453 [CI95% = 13.1%–22%]), while the rectal area was colonized in 13.8% (63/453 [CI95%= 10.8%–16.8%]) of pregnant women. Collection site stratification showed that 4.8% (22/453) of pregnant women only tested positive in the rectal area, 8.6% (39/453) only tested positive in the vaginal area, and 9% (41/453) showed colonization at both areas.
Susceptibility to antimicrobial agents was tested in 85 of the viable isolates after cryopreservation in glycerol. A total of 77.9% of the investigated isolates were resistant to at least one of the antimicrobial agents tested, with tetracycline and erythromycin showing the highest percentages of resistance of 74.1% (63/85) and 14.1% (12/85), respectively. The clindamycin resistance rate was 3.5% (3/85), and all isolates were susceptible to penicillin, ampicillin, cefazolin, and ceftriaxone. The level of resistance was evaluated at intervals over the study period. A stable rate of resistance to tetracycline was observed, while the rate of resistance to clindamycin fell and resistance to erythromycin increased considerably (Figure 2). There was no statistical difference in the antimicrobial susceptibility profiles between isolates derived from different anatomical collection areas.
DiscussionThis study represents the first effort to ascertain the prevalence of GBS colonization among pregnant women in Porto Velho, Rondônia, located in the Amazon region of Brazil. Additionally, it evaluates the sociodemographic and clinical characteristics associated with GBS colonization, as well as the susceptibility of the isolated samples to the antimicrobials commonly employed in prophylactic treatment. Research on GBS in pregnant women is particularly significant due to the fact that vaginal and/or rectal colonization by GBS constitutes the primary risk factor for neonatal GBS infection. Newborns, possessing an immature immune system, are consequently more vulnerable to neonatal sepsis, pneumonia, and meningitis caused by GBS.
15This study showed a 22.5% prevalence of GBS colonization in women between 35-37 gestational weeks. The rate of GBS colonization determined by previous studies of Brazilian patients was 4.2–28.4%.
6 The prevalence observed in this study was within the upper limit of this range. Brazil is a country of continental proportions and has intrinsic socio-regional differences, which may explain the large range in colonization rates. Another important factor that likely influences this variation is the absence of official recommendations by the Brazilian Unified Health System (SUS – Portuguese acronym) and of universal guidelines for GBS screening.
6,9The study indicated that colonization rates for maternal GBS in pregnant women from the Public Healthcare Network of the city of Porto Velho were higher than worldwide estimates (18% [CI95%= 17%–19%]), but remained within regional averages (11%–35%). South Asia and Eastern Asia had the lowest rates of GBS colonization (13% and 11%, respectively). The prevalence in this study was higher than South America's (15.9% [CI95%= 13.5%–18.2%]) and similar to those of Australia and New Zealand (23.3%), North America (23.2%), Northern Europe (22.2%), Eastern Europe (23%), and Northern Africa (22.9%).
1The colonization rate varies depending on geographic area, genetic differences in host response, sampling methodologies, and the processing protocol adopted for the screening procedure. Moreover, collection time during pregnancy, the use (or not) of enriched selective culture media, and whether the identification methodology is based on serology, molecular biology, or presumptive tests may also contribute to the variability reported in previous studies.
15In addition to the general prevalence of colonization, we also evaluated the differences in GBS load in pregnant women in relation to areas of collection and the time of study. No significant fluctuations were observed in the general colonization rates over the study period, as has previously been observed in Brazil.
16 Unlike most previous studies, which used a combined swab technique, this study used separate swabs to determine colonization in different anatomical locations, allowing rates to be stratified according to collection site. A higher rate of GBS colonization was observed in the vaginal region, with a marked growth trend over the study period. These data indicate a greater affinity of GBS to the vaginal region, which supports the findings that GBS has numerous surface adhesins and invasins that interact directly with the vaginal epithelium and promote persistent colonization in this niche.
17 Previous studies have disagreed on the superiority of vaginal colonization compared with rectal colonization, with this divergence influenced by the characteristics of the study population itself as well as the identification method used, cited above.
18,19The USA CDC recommends universal screening using culture-based methods and subsequent treatment with IAP where colonization is detected. An alternative approach uses risk factors to make decisions about IAP treatment when the colonization status is unknown.
11 Currently, there is no international consensus on which of these two approaches is more effective; however, numerous studies have shown better prophylactic coverage of neonates susceptible to GBS disease using culture screening.
7,20,21 A previous study showed a significant drop in the risk of developing early onset disease among newborns from screened pregnant women compared with that observed in those treated according to risk factors.
11 A study conducted in Rio de Janeiro, Brazil, reported that 14% of women known to be colonized with GBS using the culture-based approach would have been excluded from the IAP recommendation if only risk factors had been considered.
16Currently, Brazil does not adopt systematic and standardized universal screening for pregnant women despite global evidence of its efficacy. CDC in the US has shown an 80% decrease in early onset disease as a result of prophylaxis treatment in pregnant women.
11 In countries where there is no recommendation for IAP, there is a 1.1% likelihood of early onset disease development due to GBS colonization in pregnant women, whereas in countries that adopt prophylaxis the risk falls to 0.03%.
22 However, IAP has not affected the disease caused by GBS before childbirth or late onset disease, and there are concerns about effects on the composition of the neonatal and maternal microbiome through selective pressure and the development of antimicrobial resistance.
15,23 As evidence suggests, until more preventative strategies are available, such as a maternal GBS vaccine, universal culture-based screening in conjunction with IAP remains the most effective protocol to prevent neonatal GBS disease.
2,5In this study, sociodemographic and obstetric factors were not found to be significantly associated with GBS colonization, indicating a homogeneous distribution of GBS colonization in pregnant women in the region, as observed in previous studies conducted in Brazil and other countries.
24,25 Sociodemographic and obstetric factors may increase the likelihood of GBS colonization. Previous studies demonstrated that certain ethnic groups, ages, and specific obstetric conditions are at a greater risk of GBS colonization, as well as associated risk factors for developing early onset disease and strains with hypervirulent profiles.
2,16,26 We identified a trend towards a higher rate of colonization (though not statistically significant) in pregnant women under 20 years of age. Thus, our results demonstrate the need for universal screening of pregnant women owing to the homogeneous distribution of GBS colonization across widely varying population characteristics. This highlights the importance of continuous surveillance for GBS in the region, since the characteristics associated with colonization may change over time.
Although IAP is the main defense against early GBS infection, non-susceptibility to the antimicrobial agents commonly used in prophylaxis has been reported. Our results showed that 14.1% of the isolates were resistant to erythromycin and that the resistance rate increased over the study period, while 3.5% were resistant to clindamycin. Barros
27 highlighted that the non-susceptibility rate to clindamycin ranged from 1.9 to 18.8% and to erythromycin ranged from 4 to 25% in Brazilian studies carried out in recent decades, with a significant increase after 2010. It is noteworthy that although the degree of susceptibility to erythromycin is commonly assessed in GBS, this antimicrobial is no longer used as an option for IAP due to its pharmacokinetic properties and increasing resistance.
27,28 These temporal trends in antimicrobial susceptibility show the need for continued surveillance of resistance rates to assess whether or not these drugs can be used in IAP in the local context.
The antimicrobial agent with the highest rate of resistance observed in the present study was tetracycline, which is not recommended for use in IAP; however, its resistance levels are being monitored. Other studies carried out in Brazil have also reported high rates of resistance to this antimicrobial agent.
16,27 Additionally, high rates of resistance (>70%) have been reported in other countries.
25,29All GBS samples evaluated were susceptible to the first-choice antimicrobial agents penicillin and ampicillin, as well as to cephalosporins. Despite the reports of isolates with decreased susceptibility to penicillin in other countries, there are no reports of β-lactam-resistant GBS in Brazil so far, indicating that the first-line antibiotics recommended for IAP remain good options for the prevention of GBS neonatal disease.
24,27,30 However, this information may be underreported because penicillin-reduced susceptibility cannot be detected by agar diffusion methods, but only by minimum inhibitory concentration (MIC) tests.
27As a limitation of the present study, results relating to antimicrobial susceptibility profiling were obtained from 83.3% (85/102) of the isolates recovered from pregnant women. The preliminary identification and the isolation from clinical samples were not performed simultaneously with the characterization of the isolates, therefore some GBS strains were lost during storage period, situation already described.
16 Despite the high sensitivity and specificity of the PCR assay used to identify GBS, the lack of culture confirmation of all PCR results (both positive and negative) and the non-use of MIC to determine reduced penicillin susceptibility are also recognized as potential limitations of the study.
The findings from this study emphasize the significance of identifying maternal colonization by GBS in the local area as a means of decreasing instances of neonatal infection caused by these bacterial pathogens, due to their prevalence in the region. Furthermore, studies that continue to advance the understanding of epidemiological factors, virulence, and antimicrobial susceptibility of GBS isolates from pregnant women and neonates from the Amazon States of Brazil are crucial for developing surveillance and prevention techniques that target GBS.
This is the first study to investigate GBS colonization in this region. This study revealed a prevalence of GBS colonization in pregnant women in the Public Healthcare Network in Porto Velho, Rondônia, using both microbiological and molecular methodologies. There was no association between sociodemographic and clinical characteristics and colonization, which supports the need for uniform examinations in all pregnant women between the 36
th and 37
th gestational weeks. Moreover, though the isolates had a high rate of resistance to tetracycline and erythromycin, they also showed high susceptibility to the first-line antimicrobials used in prophylaxis. Considering that Brazil does not adopt systematic and standardized universal screening, despite global evidence of its efficacy, this study provides crucial data for the design of strategies to prevent invasive GBS infection and, consequently, minimize the mortality and morbidity caused by this pathogen in Brazil.
References1. Russell NJ, Seale AC, O'Driscoll M, O'Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J,
et al.; GBS Maternal Colonization Investigator Group. Maternal colonization with group B
Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis. 2017 Nov; 65 (Suppl. 2): S100-11.
2. Bartlett AW, Smith B, George CR, McMullan B, Kesson A, Lahra MM,
et al. Epidemiology of Late and Very Late Onset Group B Streptococcal Disease: Fifteen-Year Experience From Two Australian Tertiary Pediatric Facilities. Pediatr Infect Dis J. 2017 Jan; 36 (1): 20-4.
3. Kohli-Lynch M, Russell NJ, Seale AC, Dangor Z, Tann CJ, Baker CJ, Bartlett L,
et al. Neurodevelopmental Impairment in Children After Group B Streptococcal Disease Worldwide: Systematic Review and Meta-analyses. Clin Infect Dis. 2017 Nov; 65 (Suppl. 2): S190-9.
4. Madrid L, Seale AC, Kohli-Lynch M, Edmond KM, Lawn JE, Heath PT,
et al.; Infant GBS Disease Investigator Group. Infant group B Streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin Infect Dis. 2017 Nov; 65 (Suppl. 2): S160-72.
5. World Health Organization (WHO). Group B streptococcus full value of vaccine assessment. Geneva: WHO; 2021. [access in 2022 Nov 18]. Available from:
https://www.who.int/teams/immunization-vaccines-andbiologicals/immunizationanalysis-and-insights/vaccine-impact-value/group-bstreptococcus-full-value-of-vaccineassessment6. Nascimento CS, Santos NFB, Ferreira RCC, Taddei CR.
Streptococcus agalactiae in pregnant women in Brazil: prevalence, serotypes, and antibiotic resistance. Braz J Microbiol. 2019; 50 (4): 943-52.
7. Schrag SJ, Zell ER, Lynfield R, Roome A, Arnold KE, Craig AS,
et al; Active Bacterial Core Surveillance Team. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med. 2002 Jul; 347 (4): 233-9.
8. Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 797. Obstet Gynecol. 2020 Feb; 135 (2): e51-e72. Erratum in: Obstet Gynecol. 2020 Apr; 135 (4): 978-9.
9. Ministério da Saúde (BR). Secretaria de Atenção à Saúde. Departamento de Atenção Básica Cadernos de atenção Básica: Atenção ao Pré-Natal de Baixo Risco. Brasília (DF): Ministério da Saúde; 2023. [access in 2024 May 16]. Available from:
https://bvsms.saude.gov.br/bvs/publicacoes/atencao_pre_natal_baixo_risco.pdf10. Brazilian Institute of Geography and Statistics (IBGE). Census. 2010 [access in 2022 January 24]. Available from:
http://www.ibge.gov.br/home/estatistica/populacao/censo2010/default.shtm11. Verani JR, McGee L, Schrag SJ; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010 Nov; 59 (RR-10): 1-36.
12. Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory; 1989.
13. Ke D, Ménard C, Picard FJ, Boissinot M, Ouellette M, Roy PH,
et al. Development of conventional and real-time PCR assays for the rapid detection of group B streptococci. Clin Chem. 2000; 46 (3): 324-31.
14. European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters European Committee on Antimicrobial Susceptibility Testing. Version 11.0. 2021. [access in 2022 Jan 20]. Available from:
https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf15. Steer PJ, Russell AB, Kochhar S, Cox P, Plumb J, Gopal Rao G. Group B streptococcal disease in the mother and newborn-A review. Eur J Obstet Gynecol Reprod Biol. 2020 Sep; 252: 526-33.
16. Botelho ACN, Oliveira JG, Damasco AP, Santos KTB, Ferreira AFM, Rocha GT,
et al.
Streptococcus agalactiae carriage among pregnant women living in Rio de Janeiro, Brazil, over a period of eight years. PLoS One. 2018 May; 13 (5): e0196925.
17. Brokaw A, Furuta A, Dacanay M, Rajagopal L, Adams Waldorf KM. Bacterial and host determinants of group B Streptococcal vaginal colonization and ascending infection in pregnancy. Front Cell Infect Microbiol. 2021 Sep; 11: 720789.
18. Dashtizade M, Zolfaghari MR, Yousefi M, Nazari-Alam A. Antibiotic susceptibility patterns and prevalence of
Streptococcus agalactiae rectovaginal colonization among pregnant women in Iran. Rev Bras Ginecol Obstet. 2020 Aug; 42 (8): 454-9.
19. Nkembe NM, Kamga HG, Baiye WA, Chafa AB, Njotang PN.
Streptococcus agalactiae prevalence and antimicrobial susceptibility pattern in vaginal and anorectal swabs of pregnant women at a tertiary hospital in Cameroon. BMC Res Notes. 2018 Jul; 11 (1): 480.
20. Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B
streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine. 2013 Aug; 31 (Suppl. 4): D20-6.
21. Homer CS, Scarf V, Catling C, Davis D. Culture-based versus risk-based screening for the prevention of group B streptococcal disease in newborns: a review of national guidelines. Women Birth. 2014 Mar; 27 (1): 46-51.
22. Cho CY, Tang YH, Chen YH, Wang SY, Yang YH, Wang TH,
et al. Group B Streptococcal infection in neonates and colonization in pregnant women: An epidemiological retrospective analysis. J Microbiol Immunol Infect. 2019 Apr; 52 (2): 265-72.
23. Prescott S, Dreisbach C, Baumgartel K, Koerner R, Gyamfi A, Canellas M,
et al. Impact of Intrapartum Antibiotic Prophylaxis on Offspring Microbiota. Front Pediatr. 2021 Dec 10; 9: 754013.
24. Rocha JZ, Feltraco J, Radin V, Gonçalves CV, Silva PEA, Von Groll A.
Streptococcus agalactiae colonization and screening approach in high-risk pregnant women in southern Brazil. J Infect Dev Ctries. 2020; 14 (4): 332-40.
25. Gizachew M, Tiruneh M, Moges F, Adefris M, Tigabu Z, Tessema B.
Streptococcus agalactiae from Ethiopian pregnant women; Prevalence, associated factors and antimicrobial resistance: Alarming for prophylaxis. Ann Clin Microbiol Antimicrob. 2019; 18 (1): 1-9.
26. Zaleznik DF, Rench MA, Hillier S, Krohn MA, Platt R, Lee ML,
et al. Invasive disease due to group B
Streptococcus in pregnant women and neonates from diverse population groups. Clin Infect Dis. 2000 Feb; 30 (2): 276-81.
27. Barros RR. Antimicrobial resistance among beta-hemolytic streptococcus in Brazil: an overview. Antibiotics (Basel.). 2021 Aug; 10 (8): 973.
28. Bulska M, Szcześniak P, Pięta-Dolińska A, Oszukowski P, Orszulak-Michalak D. The placental transfer of erythromycin in human pregnancies with group B streptococcal infection. Ginekol Pol. 2015 Jan; 86 (1): 33-9.
29. Burcham LR, Spencer BL, Keeler LR, Runft DL, Patras KA, Neely MN,
et al. Determinants of Group B streptococcal virulence potential amongst vaginal clinical isolates from pregnant women. PLoS One. 2019 Dec 18; 14 (12): e0226699.
30. Campo CH, Martínez MF, Otero JC, Rincón G. Vagino-rectal colonization prevalence by
Streptococcus agalactiae and its susceptibility profile in pregnant women attending a third-level hospital. Biomedica. 2019; 1; 39 (4): 689-98.
Author's contributionCarvalho AG: methodology, formal analysis and investigation, writing - original draft preparation, writing of the final version of the manuscript.
Rodrigues RS: writing - original draft preparation, writing of the final version of the manuscript.
Rodrigues MD: conceptualization, methodology, writing - review and editing; writing of the final version of the manuscript.
Oliveira LP, Ricarte MJVG, Dorneles NWS, Lima NCS: methodology, writing of the final version of the manuscript.
Belem MGL: methodology, formal analysis and investigation, writing of the final version of the manuscript.
Rocha PRDA, Pinto TCA: writing - review and editing, writing of the final version of the manuscript.
Lima CM: conceptualization, writing of the final version of the manuscript.
Watanabe M: formal analysis and investigation, writing of the final version of the manuscript.
Taborda RLT: conceptualization, methodology, formal analysis and investigation, funding acquisition, supervision, writing of the final version of the manuscript.
Matos NB: conceptualization, methodology, formal analysis and investigation, writing - review and editing, funding acquisition, supervision, writing of the final version of the manuscript.
The authors approved the final version of the article and declare no conflict of interest.
Received on February 27, 2023
Final version presented on June 4, 2024
Approved on June 19, 2024
Associated Editor: Leila Katz
 ; Renata Santos Rodrigues2
; Renata Santos Rodrigues2 ; Mariana Delfino Rodrigues3
; Mariana Delfino Rodrigues3 ; Leticia Pereira de Oliveira4
; Leticia Pereira de Oliveira4 ; Mayra Gyovana Leite Belém5
; Mayra Gyovana Leite Belém5 ; Michelle Juliana Vieira Gomes Ricarte6
; Michelle Juliana Vieira Gomes Ricarte6 ; Nagilla Wynne dos Santos Dorneles7
; Nagilla Wynne dos Santos Dorneles7 ; Paulo Ricardo Dell'Armelina Rocha8
; Paulo Ricardo Dell'Armelina Rocha8 ; Núcia Cristiane da Silva Lima9
; Núcia Cristiane da Silva Lima9 ; Claudete Martins Lima10
; Claudete Martins Lima10 ; Michel Watanabe11
; Michel Watanabe11 ; Tatiana de Castro Abreu Pinto12
; Tatiana de Castro Abreu Pinto12 ; Roger Lafontaine Mesquita Taborda13
; Roger Lafontaine Mesquita Taborda13 ; Najla Benevides Matos14
; Najla Benevides Matos14