ABSTRACT
INTRODUCTION: cystic fibrosis newborn screening must enable its earlier diagnosis, which may enhance outcomes. This study was a series case of delayed-diagnosis children submitted to cystic fibrosis newborn screening.
DESCRIPTION: fourteen children were included; eight (57.1%) were due to false-negative screening, while six (42.9%) were due to processing errors. Two samples collected after 30 days of life were incorrectly classified as negative, and four infants with a positive test could not be located due to screening processing errors. Cystic fibrosis diagnosis was confirmed at a median (IQR) age of 5.3 (4.2-7.4) months. Poor nutritional status was the most prevalent clinical sign at diagnosis, being present in 78.6% of infants. The mean (SD) weight-for-length and length-for-age Z-scores were -3.46 (0.84) and -3.99 (1.16), respectively. Half of the children had Pseudo-Bartter syndrome, and 42.9% had breathing difficulties. Twelve children (85.7%) required hospitalization, with a median (IQR) length of stay of 17.0 (11.5-26.5) days.
DISCUSSION: newborn screening had some faults, from incorrect collections to inefficient active search. Early identification of these children in which screening was unsatisfactory is essential, emphasizing the importance and efforts to not miss them. In the case of a failed test, healthcare professionals must be prepared to recognize the main symptoms and signs of the disease.
Keywords:
Cystic fibrosis, Newborn screening, Public health policy, Delayed diagnosis, Diagnostic errors
RESUMO
INTRODUÇÃO: a triagem neonatal para fibrose cística deve contribuir para diagnóstico precoce e melhor prognóstico da doença. O estudo é uma série de casos com lactentes submetidos à triagem, porém com diagnóstico tardio da doença.
DESCRIÇÃO: quatorze crianças foram incluídas; oito (57,1%) com triagem falso-negativo e seis (42,9%) com erros processuais na triagem neonatal. Duas amostras foram coletadas tardiamente, sendo incorretamente classificadas como negativas e quatro lactentes com triagem positiva não foram localizados, por erros na busca ativa. Confirmou-se o diagnóstico da fibrose cística com idade mediana (IIQ) de 5,3 (4,2-7,4) meses. O Comprometimento nutricional precoce foi o sinal clínico mais prevalente ao diagnóstico, presente em 78,6% das crianças. Os Z escores médios (SD) do peso para altura e altura para idade foram -3,46 (0,84) e -3,99 (1,16), respectivamente. Metade das crianças teve síndrome de Pseudo-Bartter e 42,9% dificuldade respiratória. Doze crianças (85,7%) precisaram hospitalização com tempo mediano de permanência de 17 dias.
DISCUSSÃO: a triagem neonatal para fibrose cística apresentou falhas, desde testes falso-negativos, coletas incorretas, até problemas com a busca ativa. Entretanto, o diagnóstico ágil é essencial e os profissionais de saúde devem reconhecer os sintomas e sinais precoces da doença, mesmo quando a triagem neonatal não for satisfatória.
Palavras-chave:
Fibrose cística, Triagem neonatal, Política pública de saúde, Diagnóstico tardio, Erro diagnóstico
IntroductionCystic fibrosis (CF) is an inherited autosomal recessive genetic disorder caused by the presence of two pathogenic variants in the
cystic fibrosis transmembrane conductance regulator (
CFTR)
gene that encodes the CFTR protein. The disease is more prevalent among caucasian populations. CF is chronic and progressive, affecting multiple organs and diminishing life expectancy and quality of life.
1 The advent of CF newborn screening (NBS) enabled diagnosis to be made at an earlier stage, thus contributing towards improving short- and long-term outcomes.
2In 1979, Crossley
3 detected high levels of immunoreactive trypsinogen (IRT) in patients with CF, thus laying the cornerstone for the development of NBS. Increased IRT levels are indicative of pancreatic injury and suggestive, but not confirmatory, of CF.
3Worldwide, IRT measurement is the initial step in the various CF NBS protocols. The implementation of NBS has been contributing to the early diagnosis of CF, preferably prior to two months of life, at which time few or no symptoms of the disease are present.
1,4 Nonetheless, errors may occur during the processes required to diagnose CF in a newborn, either due to false-negative results or processing errors during the NBS method.
The adoption of well-defined protocols for operationalizing NBS tests is aimed at preventing technical and human errors and incorporating enhancements to the adopted models to improve the test's sensitivity and specificity. Nevertheless, there will still be a certain percentage of children with false-negative results at CF NBS.
1,4The goal NBS is to diagnose diseases at early stage, preferably asymptomatic, when treatment may be more effective.
1 Numerous health professionals must collaborate to ensure all stages work properly. NBS programs are complex and involve federal, state, and municipal agencies, at a government cost.
1The National Neonatal Screening Program (NNSP) encompasses all 26 states and the Federal District of Brazil, which was established in 2001 as a component of the Brazilian Unified Health System (SUS).
1 The initial NNSP consisted of three distinct stages: screening for phenylketonuria and congenital hypothyroidism, sickle cell anemia and other hemoglobinopathies, and cystic fibrosis. Congenital adrenal hyperplasia and biotinidase deficiency became part of the NNSP in 2014.
1 Congenital toxoplasmosis joins the NNSP, resulting in seven diseases investigated.
5This study describes clinical data of children with CF born between 2013 and 2019 who were submitted to CF NBS and had a delayed diagnosis (> 2 months of life) because of a false-negative screening or NBS processing errors. The children were followed up at one of CF care centers of the state. The evaluation comprised a descriptive analysis of registry data from the CF care center and newborn screening service reference center (NBSRC).
The study is part of a major project entitled "Neonatal screening for cystic fibrosis in the state of Bahia, Brazil: evaluation of the first five years", which part of the data has already been published.
6DescriptionCF NBS was implemented in Bahia in March 2013, following the protocol proposed by the Ministry of Health and the Brazilian Cystic Fibrosis Study Group. The protocol consists of two separate IRT tests (IRT/IRT), with a cut-off point of 70 ng/ml.
7 Samples of IRT1 or IRT2 from newborns older than 30 days are deemed inadequate and unreliable, because the IRT concentration tends to be lower after this period, increasing the number of false-negative.
1,4,7 Children with two elevated samples of IRT high or those older than one month with only a single positive IRT1 (70 ng/ml) must be referred to a specialized center to investigate CF.
7This study is a retrospective case series that included infants born between 2013 and 2019, with CF who underwent CF NBS in the public healthcare system but were diagnosed later due to a false-negative screening test result or processing errors during screening. The group of children were from at the CF care center of a university teaching hospital.
False-negative CF screening was defined as: (1) negative IRT1, clinical status compatible with CF, and positive sweat test (ST)
or (2) positive IRT1 (≥70ng/ml), negative IRT2 (<70 ng/ml), clinical status compatible with CF, and positive ST.
CF NBS processing errors consisted of: (1) IRT1 elevated (≥70 ng/ml), but the individual failed to return for IRT2 collection or the ST;
or (2) IRT1 and IRT2 elevated (≥ 70 ng/ml) however, the individual did not attend the screening center for the ST;
or (3) IRT1 or IRT2 performed after 30 days of life (invalid samples).
All data on outpatient and inpatient follow-up were collected from electronic health records of these infants. Data entry and analysis were performed using Microsoft Excel
® for Mac. A descriptive analysis of the study population was conducted using measures of central tendency (means, medians) and dispersion (standard error and interquartile range) for the quantitative variables and measures of absolute and relative frequency for the categorical variables.
At the time of analysis, 32 individuals with a diagnosis of CF had undergone CF NBS between 2013 and 2019 and were being seen at the CF care center. Fourteen children were included in this study. All of them had delayed CF diagnosis, in eight cases (57.1%) due to false-negative results and in six cases (42.9%) due to processing errors during NBS. Eight (57.1%) children were male, while 10 (71.4%) were born outside the state capital. The CF diagnosis was confirmed by the elevation of sweat chloride and/or the identification of two pathogenic
CFTRgene variants at a median (IQR) age of 5.3 (4.2–7.4), ranging between 2.3 and 8.2 months.
At the admission to the CF care center, 11 infants (78.6%) had poor nutritional status, with this being the most common clinical symptom. Five (45.5%) of these 11 infants suffered from malnutrition, which in 3 of them (60%) was severe. Seven (63.6%) were stunted, and one suffered from both malnutrition and short stature. The mean weight for length (W/L) Z-score (SD) for these five infants with malnutrition was − 3.46 (± 0.84), and the mean length for age (L/A) Z-score (SD) for the seven cases was − 3.99 (±1.16). Twelve infants (85.7%) required hospitalization to stabilize their clinical status, with a median (IQR) length of stay of 17.0 (5–26.5) days and a range of 5.0 to 55 days. Seven infants (50%) had pancreatic insufficiency.
In addition to nutritional issues, metabolic, respiratory symptoms and liver failure were present in seven (50.0%), six (42.9%) and two (13.4%) infants, respectively. All infants with Pseudo-Bartter syndrome (hypochloremic metabolic alkalosis) required hospitalization to stabilize their condition. Meconium ileus was diagnosed in one child who underwent surgery to treat this condition. All clinical and laboratory data of the 14 patients are shown in Table 1.
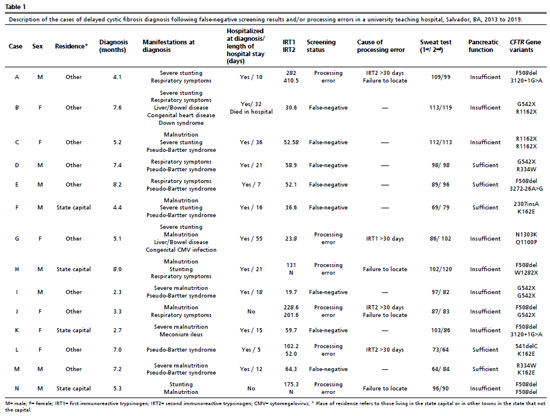
The causes of processing errors at the CF NBS included four instances in which the samples (IRT1 or IRT2) were collected after 30 days of life. These results were normal for two of them. However, both children should have been referred to a CF care center to perform the ST. In the first case, there was a negative result for IRT1, and in the second case, the IRT1 was elevated, but the IRT2 was negative. The remaining two cases also had samples (IRT2) collected 30 days after birth, but with positive results. In those occasions, the active search effort to establish communication with the parents, at an appropriate time, proved unsuccessful. In addition to the aforementioned four cases, there were two children who exhibited increased levels of IRT1. Despite the intensive attempts to look for the parents to bring the children to the CF care center, all of this proved ineffective.
One newborn with meconium ileus, whose blood sample was not sent for analysis until 28 days after collection, and two others for whom the significance of the detected variant in the CFTR gene was uncertain were among the eight children with false-negative results.
8 Each individual's clinical and laboratory information, as well as the reasons for the delay in CF diagnosis, are detailed in Table 1.
All the children were submitted to investigation for pathogenic variants and/or full-gene
CFTR sequencing, with 12 different variants being identified. The allele frequencies of variants are described in Table 2.
DiscussionThis study describes cases in which CF NBS was ineffective, highlighting the challenges experienced in these instances and the potential early consequences of undetected CF. Due to the clinical severity of their disease, almost 90% of babies were hospitalized at diagnosis. The median length of stay at the hospital was 17 days, and one infant passed away. The median age of diagnosis was older than indicated by CF NBS standards, at 5.3 months.
1These infants had early signs of both acute (malnutrition) and chronic (stunting) nutritional deficiency. Only three (21.4%) children had adequate nutritional status. As supported by other studies, poor nutritional status at a young age is associated with clinical severity,
2 and there is a clear association between stunting and the unfavorable progression of the disease, with this relationship tending to be irreversible, developing in the first years of life, and being associated with the loss of pulmonary function.
9 Therefore, nutritional status appears to be an early indicator of CF and should be regarded as an important warning sign for healthcare professionals. In this study, poor nutritional status was common at the time of the CF diagnosis, which can cause poor outcomes in the short and long term.
Other common clinical symptoms in these children included fluid and electrolyte issues, particularly Pseudo-Bartter syndrome. This syndrome can cause severe morbidity, possibly requiring hospitalization for the infant, as was the case for all infants in the study with these symptoms.
10,11 A greater proportion of infants were identified with Pseudo-Bartter syndrome than in an Italian research, which a retrospective analysis of a cohort revealed 18 cases of false-negative CF-NBS results, including seven occurrences (38.8%) of Pseudo-Bartter syndrome.
10 In another investigation, in 1992, with equivalent methodology, this disorder was detected in <13.0% of instances.
11 In fact, this manifestation appears to be influenced by the climatic conditions of the population's region as well as the allele variants present.
11 The state of Bahia has a tropical climate; therefore, temperatures are high almost all year, which may contribute to the greater prevalence of Pseudo-Bartter syndrome reported here.
Meconium ileus is a condition associated with negative screening results and is typically the first sign of cystic fibrosis (CF), requiring effective steps to improve newborn care. Even if screening tests are negative, a ST and/or DNA analysis for CFTR variants must be performed.
1,7 According to available data from Europe and North America, it has been observed that meconium ileus affects approximately 20% of individuals within the CF population.
12 In a prior study conducted in Brazil, in 2001, the estimated prevalence of meconium ileus was around 7.0%.
13 Still, the incidence rate at the CF care center, where the current investigation was conducted out, is short at approximately 3.0%. However, additional investigation is necessary to determine the underlying cause of this disparity. Underreporting and/or regional disparities in the clinical presentation of this symptom may exist and are likely to be attributed to the wide range of gene variants seen in the population.
Although IRT measurement constitutes a valid screening instrument for CF, there are some problems with this method.
4 The percentage of infants with false-negative screening results deemed acceptable has ranged from 5 to 15% in the various studies, with sensitivity being poorer with the currently implemented NBS test.
1,4 Despite these difficulties, all neonatal CF screening models implemented around the world use IRT measurement as the first step in the process.
1,4The IRT/IRT strategy depends on the availability of the health resources required to obtain a second sample for IRT2 within an appropriate time span.
4 In a recent study conducted in Turkey, in 2021, only around 70% of the infants submitted to CF NBS underwent a second IRT measurement before diagnosis was confirmed.
14 A large project of our research group utilizing data from the first five years of CF NBS Bahia State revealed that only 76.4% of the infants who tested positive for IRT1 were retested.
6 These data clearly show the difficulties involved in obtaining a second IRT measurement, particularly in a state as large as Bahia, highlighting the errors made in following up on these cases.
In two of the six cases (42.9%) where processing errors occurred during NBS, samples for IRT1 and IRT2 were collected beyond 30 days of life, with results considered negative by the NBSRC. IRT1 and/or IRT2 were positive in the remaining four instances. In these cases, the errors occurred during the localization phase, and the screening center was unable to find these infants to complete the screening procedure. Communication between the NBSRC, primary healthcare units, and the child's family is a significant barrier to early diagnosis, according to a study conducted in the United States in 2022,
15 which recognized the need for continued education of healthcare professionals and the general public about the benefits of NBS. The prior study cited previously revealed comparable problems and advocated effective strategies to overcome these obstacles.
6This case series found that the median time taken to analyze IRT1 samples was 8.5 days, with 7/14 samples (50%) collected after 10 days of life, of which one was collected after 30 days of life. Environmental factors such as the state of Bahia's high average temperature and the timing between sampling and analysis might just have contributed to false-negative reports. The study conducted by Therrel
et al.
4 in 2012 showed an important reduction in IRT measurement in dried blood spot samples stored for a week at high temperature and humidity. Delays in analyzing samples are known to affect the quality and, consequently, the test outcomes.
4 This emphasizes the significance of adequate flow in enhancing the effectiveness of a NBS program.
The degree of allelic heterogeneity in the population screened can affect the sensitivity of IRT1,
1,4 particularly when there are variants with low population frequency, as seen in this study population (12 different variants in the 14 individuals evaluated). One of these allelic variants of the
CFTRgene (
K162E) is of uncertain significance and appears in heterozygosity in three infants, all of whom were diagnosed with Pseudo-Bartter syndrome.
8 In these cases, the disease was less severe in two of the children; however, the third child, who had
Pseudomonas aeruginosa on multiple occasions, presented with pancreatic insufficiency.
This study's weaknesses include its design and the small number of participants. Nonetheless, the study was able to identify logistical issues that impair the success of the CF NBS program, such as issues with sample collection and locating children with elevated IRT.
This paper highlights the significance of improving the CF NBS procedures in order to reduce the incidence of false negatives and missing cases. The parent's contact information, such as phone number and address, should be registered with care, since they are required for an effective active search that reduces errors. Health professionals must be continually trained and reminded of the importance of NBS and its timely execution. Recognizing delayed CF diagnosis scenarios is a critical step toward understanding and improving the whole healthcare system and public policies involved with the NBS program. It is essential that health workers should be able to diagnose the most common clinical manifestations in children with CF, even if NBS results are negative, avoiding long-term consequences of the disease.
Based on this study, the prevention of delayed CF diagnosis was not satisfactorilly achieved and there are still aspects of the screening that need to be improved. The NBS for CF is an important Public Health Program which must be continuously assessed.
References1. Ministério da Saúde (BR). Secretaria de Atenção a Saúde. Departamento de Atenção Especializada e Temática. Triagem Neonatal Biológica. Manual Técnico. 1
st ed. Brasília (DF): Ministério da Saúde; 2016. [access in 2023 Mai 6]. Available from:
https://bvsms.saude.gov.br/bvs/publicacoes/triagem_neonatal_biologica_manual_tecnico.pdf2. Tridello G, Castellani C, Meneghelli I, Tamanini A, Assael BM. Early diagnosis from newborn screening maximises survival in severe cystic fibrosis. ERJ Open Res. 2018 Apr; 4 (2): 00109-2017.
3. Crossley JR, Smith PA, Edgar BW, Gluckman PD, Elliott RB. Neonatal screening for cystic fibrosis, using immunoreactive trypsin assay in dried blood spots. Clin Chim Acta. 1981 Jun; 113 (2): 111-21.
4. Therrell BL, Hannon WH, Hoffman G, Ojodu J, Farrell PM. Immunoreactive trypsinogen (IRT) as a biomarker for cystic fibrosis: challenges in newborn dried blood spot screening. Mol Genet Metab. 2012 May; 106 (1): 1-6.
5. Ministério da Saúde (BR). Portaria GM/MS No 1.369, de 6 de Junho de 2022. Altera e inclui procedimento relacionado a Triagem Neonatal na Tabela de Procedimentos, Medicamentos, Órteses, Próteses e Materiais Especiais (OPM) do Sistema Único de Saúde (SUS) e estabelece recurso do Bloco de Manutenção das Ações e Serviços Públicos de Saúde - Grupo de Atenção Especializada, a ser incorporado ao limite financeiro de Média e Alta Complexidade (MAC), de Estados. Brasília (DF): Ministério da Saúde; 2022. [
Internet]. [access in 2023 Mai 6]. Available from:
https://bvsms.saude.gov.br/bvs/saudelegis/gm/2022/prt1369_08_06_2022.html6. Godoy C, Paixão DC, Boa-Sorte NCA, Amorim T, Silva Filho LVRF, Souza EL. Five-year performance analysis of a cystic fibrosis newborn screening program in northeastern Brazil. J Pediat (Rio J). 2023; 99 (1): 23-30.
7. Athanazio RA, Silva Filho LVRF, Vergara AA, Ribeiro AF, Riedi CA, Procianoy EFA,
et al. Brazilian guidelines for the diagnosis and treatment of cystic fibrosis. J Bras Pneumol. 2017; 43 (3): 219-45.
8. Souza EL, Mota LR, Lima RLLF, Bittencourt PH, Guedes VMCR, Salinas D. K162E - A rare and uncategorized CFTR variant causing cystic fibrosis. J Cyst Fibros. 2021 May; 20 (3): 489-91.
9. Marks MP, Heltshe SL, Baines A, Ramsey BW, Hoffman LR, Stalvey MS. Most Short Children with Cystic Fibrosis Do Not Catch Up by Adulthood. Nutrients. 2021 Dec; 13 (12): 4414.
10. Taccetti G, Botti M, Terlizzi V, Cavicchi MC, Neri AS, Galici V,
et al. Clinical and genotypical features of false-negative patients in 26 years of cystic fibrosis neonatal screening in Tuscany, Italy. Diagnostics (Basel). 2020 Jul; 10 (7): 446.
11. Davis SL, Gunn TR, Tonkin SL, Hadden W. Genetic and clinical features of false-negative infants in a neonatal screening programme for cystic fibrosis. Acta Paediatr. 2002; 91 (1): 82-7.
12. Sathe M, Houwen R. Meconium ileus in Cystic Fibrosis. J Cyst Fibros. 2017 Nov; 16 (Supl. 2): S32-9.
13. Oliveira MCLA, Reis FJC, Monteiro APAF, Penna FJ. Effect of meconium ileus on the clinical prognosis of patients with cystic fibrosis. Braz J Med Biol Res. 2002; 35 (1): 31-8.
14. Gokdemir Y, Eyuboglu TS, Emiralioglu N, Er B, Sen V, Pekcan S,
et al. Geographical barriers to timely diagnosis of cystic fibrosis and anxiety level of parents during newborn screening in Turkey. Pediatric Pulmonol. 2021 Oct; 56 (10): 3223-31.
15. Sontag MK, Miller JI, McKasson S, Gaviglio A, Martiniano SL, West R,
et al. Newborn Screening for Cystic Fibrosis: A Qualitative Study of Successes and Challenges from Universal Screening in the United States. Int J Neonatal Screen. 2022 Jun; 8 (3): 38.
Acknowledgements: We would like to thank the Foundation for the Support of Research in the State of Bahia (
Fundação de Apoio à Pesquisa do Estado da Bahia - FAPESB) and the Brazilian Group of Studies on Cystic Fibrosis (
Grupo Brasileiro de Estudos sobre Fibrose Cística - GBEFC).
Author's contribution: Godoy C: conception, analysis, investigation, methodology, supervision, validation, interpretation of data and writing of draft and review of the article; Radel I: data curation, analysis, investigation, validation, visualization and writing original draft of the article; Mota LR: investigation, validation, visualization and writing and review the article; Santos MA: conception, analysis, validation, and visualization of the article; Terse-Ramos R: conception, planning methodology, validation, visualization and writing and review of the article; Souza EL: conception, analysis, investigation, resources, supervision, interpretation of data and writing of draft and review of the article.
All authors approved the final version of the article and declare that have any conflict of interest.
Received on July 24, 2023
Final version presented on November 19, 2023
Approved on November 23, 2023
Associated Editor: Karla Bomfim
 ; Igor Radel2
; Igor Radel2 ; Laís Ribeiro Mota3
; Laís Ribeiro Mota3 ; Marília Augusta Santos4
; Marília Augusta Santos4 ; Regina Terse5
; Regina Terse5 ; Edna Lúcia Souza6
; Edna Lúcia Souza6