ABSTRACT
OBJECTIVES: To evaluate the medication use, exposure to potential risks, and associated factors before and during pregnancy of pregnant women receiving care at the Family Health Strategy in a municipality in the Northeast of Brazil.
METHODS: This is a cross-sectional study of pregnant women receiving care in the municipality of Barreiras, in Bahia, Brazil. In data analysis process, prevalence and frequency of medication use were estimated. To investigate the association between variables, the outcome measure was expressed by the prevalence ratio (crude and adjusted) with a 95% confidence interval via Poisson regression.
RESULTS: The prevalence of medication use before pregnancy was 35% and during pregnancy, it was 80.7%. Analgesics and antianemics were the prevalent groups of medications before and during pregnancy, respectively. Family income (≤1 minimum wage; PR=1.62; CI95%=1.02-2.55) showed an association with prior use; health problems (PR=2.3; CI95%=1.27-4.22) and complaints in pregnancy (PR=2.39; CI95%=1.28-4.47) had an association with use during pregnancy.
CONCLUSIONS: The characterization of a high prevalence of use of medicines by pregnant women, combined with a trend of failures in family planning could demonstrate the exposure of the risks of using some harmful substances in periods close to conception and pregnancy.
Keywords:
Drug utilization, Pregnant women, Pharmacoepidemiology, Prenatal care
RESUMO
OBJETIVOS: avaliar o uso de medicamentos, exposição a potenciais riscos e os fatores associados antes e durante a gestação pelas gestantes atendidas na Estratégia Saúde da Família em município do nordeste brasileiro.
MÉTODOS: trata-se de um estudo transversal realizado com gestantes atendidas no município de Barreiras, Bahia, Brasil. No processo de análise dos dados, foram estimadas as prevalências e frequências de utilização de medicamentos. Para investigar a associação entre variáveis, a medida do desfecho foi expressa pela razão de prevalência (bruta e ajustada) com intervalo de 95% de confiança pela regressão de Poisson.
RESULTADOS: a prevalência do uso de medicamentos antes da gestação foi 35% e durante de 80,7%. Os analgésicos e antianêmicos foram os grupos de medicamentos prevalentes antes e durante a gestação, respectivamente. A renda familiar (≤1 salário mínimo; RP=1,62; IC95%=1,02-2,55), mostrou associação ao uso anterior; problemas de saúde (RP=2,32; IC95%=1,27-4,22) e queixas na gestação (RP=2,39; IC95%=1,28-4,47) tiveram associação para o uso durante.
CONCLUSÕES: a caracterização de uma alta prevalência do uso de medicamentos por gestantes, aliado a uma tendência de falhas no planejamento familiar pôde demonstrar a exposição dos riscos da utilização de algumas substâncias nocivas em períodos próximos da concepção e na gestação.
Palavras-chave:
Uso de medicamentos, Gestantes, Farmacoepidemiologia, Cuidado pré-natal.
IntroductionIn Brazil, the structuring of a line of care in maternal and infant health,
Rede Cegonha (Stork Network), promotes the improvement of actions to widen free access to health for this group through the promotion of pre-natal care.
1 The Family Health Strategy (FHS) serves as a foundation for that policy for conceiving planning that will achieve the wellbeing of pregnant women.
2 However, often at some point in pregnancy, symptoms or discomforts can affect their health situation. This is perceived due to the physical and/or psychological changes caused by this physiological phenomenon.
3 As they belong to a medicalizing culture, most pregnant women see medication as the only option for curing pain and other common events.
4 This situation represents eminent threats, given that medication use during pregnancy can expose the fetus to teratogenic actions, fetotoxic risks, alterations in tissue development or in embryonic organ formation, and dysfunction of some previously-formed fetal structure.
5 Thus, the real problem is not only the view that pregnant women have of medication, but also the avoidable risks to which they and their children are being exposed through using medications with questionable safety.
In addition to this, since the historic accident with thalidomide in 1950 that generated congenital defects in more than 10,000 children, there has been a change in the investigation of drug use in pregnancy.
6 However, for ethical reasons, pregnant women are excluded from clinical trials, hindering the mission of determining the safety of drugs in pregnancy, thus producing an even more restricted medical practice.
7 In order to minimize the persistent doubts during possible pharmacological interventions, the Food and Drug Administration (FDA) classifies medications according to their evidence-based risks.
8Despite the dissemination of information on the potential risks of medications in pregnancy, their use by pregnant women has proven to be an increasingly common practice. As evidence of this, one multinational study, conducted among countries in Europe, North and South America, and Australia, reveals an 81% prevalence of medication use during pregnancy. Similarly, Brazilian studies have shown 80-94% prevalence of such use.
4,10 In addition, it has been highlighted that older mothers (above 30 years old) who do not have black skin and belong to a low socioeconomic level may represent the group of pregnant women that are associated with greater medication consumption in pregnancy.
4,10 Similarly, married women with a high income and higher educational levels also present that relationship with use.
11Knowledge about the safety of medication consumption in pregnancy is limited to a small amount of scientific evidence. Thus, observational epidemiological studies can help in determining which potential risks this group is exposed to, as well as evaluating the state of maternal and infant health. Such studies contribute significantly to determining the role of the FHS and health units in transforming the situations in which pregnant women are embedded with increased risks of medication use.
The present study aims to evaluate medication use, exposure to potential threats, and associated factors before and during pregnancy of pregnant women receiving care through the FHS in one municipality in the Brazilian Northeast.
MethodsThis is a cross-sectional study that forms part of a prospective cohort study entitled “Maternal-infant cohort: epidemiological profile of pregnant women, breastfeeding women, and children receiving care through Family Health Strategy in the municipality of Barreiras, Bahia.” The research was conducted at the FHS units of the municipality of Barreiras, in Bahia, in the Northeast of Brazil. The research population comprised pregnant women who received pre-natal care at the family health units (FHUs) of the Unified Health System (SUS – Portuguese acronym). The study was developed in the period from January to December of 2019.
According to the Brazilian Institute of Geography and Statistics (IBGE – Portuguese acronym), the city is located in the western region of Bahia, 863 km from the capital Salvador and 622 km from the federal capital, and had an estimated population of 155,493 people in 2019. It covers an area of 7,859,716 km² (2018) and has a population density of 17.49 inhab/km
2 (2010), as well as being crossed by three important federal highways (the BR-020, BR-135, and BR-242), characterizing it as the main crossroads for the Midwest, Northeast, and North regions of Brazil.
The municipality is the macroregional health headquarters of reference for 37 small and medium-sized municipal systems. In January of 2019, the city of Barreiras contained 26 FHUs, one located in the rural zone and the rest in the urban zone, and it had 63.24% basic healthcare coverage.
12 It had an infant mortality rate of 14.62 deaths per thousand live births, which placed it in 216
th position in the comparative ranking of cities in the state of Bahia, in 2017.
13To compose the sample, the total live births (n=2716) in the city of Barreiras in 2018 was used, which was obtained from the Information System on Live Births (SINASC – Portuguese acronym) in DATASUS. Despite it is an overestimation of the quantity of pregnant women receiving care through the FHS in the municipality, this information was used due to the lack of disclosure of the total pregnant women monitored by the FHS. The sample calculation also considered a 50% prevalence of medication use, with a maximum acceptable error of 5 percentage points and 95% confidence level. Thus, a minimum sample of 337 pregnant women was obtained.
The pregnant women were chosen randomly at the FHUs in the urban zone of the municipality, with the rural zone being excluded due to the difficulty of access. The study included pregnant women aged 18 years old or over, resident and domiciled in the urban zone of the municipality, of any gestational age, and who had carried out at least one pre-natal consultation.
A structured script was followed during the individual interviews and documentary analysis of the pregnant women’s patient cards was carried out. Information was collected on socioeconomic and maternal variables, including aspects involving the women’s health in previous pregnancies, when applicable, and data on their current pregnancy, such as medication use.
Based on the assumption that some had unplanned pregnancies, an effort was made to obtain data on medication use not only during but also before the pregnancy. For those who answered yes, the name, pharmaceutical form, and dose of each pharmaceutical product were recorded, along with whether it was prescribed or not by a health professional.
The medications self-reported by the pregnant women were classified based on the levels of the Anatomical Therapeutic Chemical Classification System (ATC), of the World Health Organization (WHO).
14 With the aim of categorizing the potential risks the mother and child were being exposed to, medications were also classified according to the categories of risk in pregnancy established by the Food and Drug Administration (FDA),
8 in which they are classified as A (studies in humans have not shown risks), B (unlike studies in humans, those conducted in animals show risks), C (no studies have been conducted in humans, but those in animals show a risk), D (evidence of human fetal risk), and X (the risks highlighted exceed any benefit); and according to the indications (indicated, contraindicated, and use with caution) of the National Health Surveillance Agency (Anvisa)
15 during the pregnancy period.
To analyze exposure to medication use, socioeconomic, demographic, maternal, and health service use variables were evaluated, including: maternal age (18-25, 26-29, 30-34, >34 years), planned pregnancy (yes, no), schooling (≤8, 9-11, >11 years), marital status (with a partner, without a partner), family income (>1, ≤1 minimum wage), economic class (A/B, C/D/E), skin color (black, non-black), smoker (yes, no), alcohol consumption (yes, no), number of previous pregnancies (≥2, <2), history of miscarriage (yes, no), start of pre-natal care (during the 1
st trimester, after the 1
st trimester), number of pre-natal consultations (≤3, >3), having any of the following health problems (yes, no): anemia, asthma, tuberculosis, pneumonia, diabetes, hypertension, kidney disease, urinary infection, and hemorrhoids; and having the following complaints in pregnancy (yes, no): nausea, vomiting, pain, fever, gases, heartburn, inflammation, constipation, headache, abdominal colic, diarrhea, and lack of appetite.
The questionnaires obtained from the interviews were entered and validated in the Validate Epidate software, version 3.1, with an automatic system for checking consistency and validity. After the validation and clearing of duplicates and systematic errors, the information on the medications was allocated into tables in the Microsoft Excel 2007 program to facilitate quantification and classification according to the pre-defined categories of the ATC, FDA, and Anvisa.
The statistical analyses were carried out in the Stata program, version 13.0 (StataCorp LP, College Station, United States).
In the data analysis process, the respective prevalence rates and frequencies of medication use before and during pregnancy were estimated, using the total pregnant women and total medications as a denominator, respectively, according to the demographic, socioeconomic, and health characteristics. The bivariate analysis was carried out to investigate the association between the independent variables and medication use before and after pregnancy; the outcome measure was expressed by the prevalence ratio (PR) with a 95% confidence interval (CI95%). For the multivariate model, the variables with
p≤0.20 were included in the crude analysis, using the stepwise procedure. It was thus possible to calculate the adjusted PR (aPR) estimated via Poisson regression with robust variance and a CI95%, with
p≤0.50.
The “Maternal-infant cohort: epidemiological profile of pregnant women, breastfeeding women, and children receiving care through the Family Health Strategy in the municipality of Barreiras, Bahia” was previously approved by the Human Research Ethics Committee of the São Francisco River University Center (UNIRIOS), under CAAE n. 32748820.1.0000.8166 and opinion n. 4,135,057.
ResultsA total of 337 pregnant women receiving care through the FHS were included in the study. Among that public, most (40.8%) were aged 18 to 25 years old and more than half (65.5%) presented a good level of schooling with more than 11 years of studies. However, almost 72% of these pregnant women had a family income of one minimum wage or less and around 80% belonged to economic classes C, D, or E. With regard to their marital status, 92.8% lived with a partner, and 57.6% had not planned their pregnancy. A large portion of the pregnant women (84.6%) already started pre-natal care in the first trimester of their pregnancy and by the day of their interview most had carried out more than three consultations (62.3%). A little more than half (52%) of the interviewees had no diagnosis of any health problem, but 80% of them had complaints during their pregnancy (Table 1).
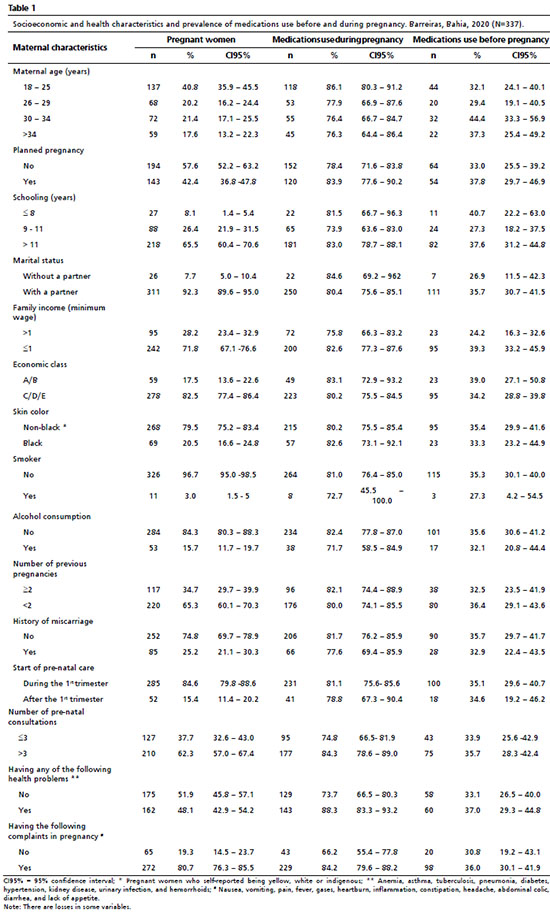
Medication use before the moment of discovering the pregnancy was self-reported by 118 (35%) women, representing a total of 173 drugs used, with participants reporting the use of more than one medication. Among these, 27% involved self-medication (data not presented in a table). According to the second level of the ATC, the most prevalent classes were analgesics (12.8%), sexual hormones and modulators of the genital system (9.2%), treatment for functional alterations of the stomach and intestines (2.7%), antianemics (2.7%), and beta-adrenergic receptor-blocking agents (1.2%) (Table 2).
During pregnancy, the number of women that used at least one medication increased to more than double, presenting a prevalence of 80.7%. In relation to the quantity of uses, the growth was greater (n=623), reaching almost four times that of the period prior to the diagnosis. Of these uses, almost all (98%) were reported as prescribed and requested by a trained professional (data not presented in a table).
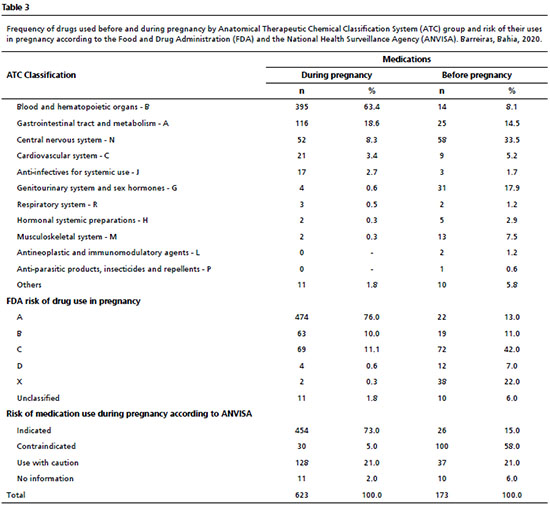
According to the first level (by anatomical group) of the ATC classification, there was a greater prevalence of women who during their pregnancy used medications for the blood and hematopoietic organs (64.4%), the gastrointestinal tract and metabolism (30.6%), the central nervous system (14.2%), the cardiovascular system (6%), and anti-infectives for systemic use (4.7%). In the second level, according to the therapeutic purpose, there was a predominance of women that used the following drugs: antianemics (64.4%), vitamins (22.3%), analgesics (14.2%), antibacterial drugs (4.5%), and antihypertensives (4.2%) (Table 2).
Most of medications used before pregnancy had a risk in pregnancy classification of C (42%) and were considered to be contraindicated for use, primarily in the first trimester (58%). After these, the most frequent were those classified with risk X (22%), A (13%), B (11%), and D (7%), with an Anvisa indication for use (15%) and to be used with caution (21%). The classification of those used during pregnancy showed that a large portion (76%) presented risk A, 11.1% presented risk C, 10% had risk B, and 0.6% and 0.3% had risk D and X, respectively. Regarding the Anvisa classification, 73% were indicated in pregnancy, 21% to be used with caution, and 5% were contraindicated (Table 3).
Among the exposure variables for medication use in the period prior to pregnancy, the only one that presented a statistically significant association in the bivariate analysis was having a family income of one minimum wage or less (PR=1.62; CI95%=1.02-2.55). For the time of pregnancy, carrying out more than three antenatal consultations (PR=1.80; CI95%=1.04-3.12), having some health problem (PR=2.68; CI95%=1.49-4.81), and the presence of complaints in pregnancy (PR=2.72; CI95%=1.48-5.00) also obtained a similar association. After the multivariate analysis, a family income ≤1 minimum wage (PR=1.63; CI95%=1.03-2.58) remained significant in the period prior to pregnancy, and only “having a health problem” (PR=2.32; CI95%=1.27-4.22) and “having a complaint in pregnancy” (PR=2.39; CI95%=1.28-4.47) remained as factors associated with medication use during pregnancy (Table 4).
DiscussionMedication use before pregnancy represents some risk to the pregnancy, primarily for women who did not intend to become pregnant, given that they did not previously imagine that their habits could be damaging the development of a future child.
16 As a result, medication use in that period was found among 35% of the pregnant women. Compared with other studies, this datum was relatively lower since they presented prevalence rates ranging from 46.7% to 52.1%.
17 In any case, this number shows maternal exposure to avoidable outcomes for both those involved. Despite the fact that medication use before pregnancy should be avoided, certain diseases require some pharmacological treatment, and so family planning and pre-conception counseling is needed to ensure safety to lives.
In addition, the quantity of medications before pregnancy without prescription was lower than the value of 53.9% found in one Brazilian study in rural Bahia.
10 However, the dangers to which women are exposed cannot be ignored, given that the dangers of self-medication are even greater. The criteria a professional uses to choose medications are different from those used in self-medication, so there is low probability of consumption of those considered to be risky if used via prescription.
11,17Before pregnancy, the analgesic class was the most used among the women, with dipyrone standing out as the most frequent. The use of this drug was associated with the development of congenital defects, such as Wilms tumor, and suggested an increased risk of leukemia in infancy.
18 After analgesics, the most used were sexual hormones, including oral contraceptives.
Population-based studies discard the idea that early fetal exposure to contraceptives is associated with congenital defects or spontaneous miscarriage.
19 Despite the evidence indicated, the prevalence of use of these drugs reflects a possible problem of therapeutic adhesion that resulted in contraceptive inefficiency. That could have been avoided with counseling from a pharmacist or the prescriber and these women may not be in favorable (health, financial, psychological, or social) conditions for an unplanned pregnancy.
In pregnancy, increased medication use represents a motivation for there to be measures regarding the care given in gestational development and in the mapping of the real needs and risks of the practice. Similarly to the present study, there is evidence that the use represents around 80% of pregnant women.
4,10 Other national studies have presented a prevalence of more than 90%.
11,16 The development of the pharmaceutical area together with government investment promotes the growth in consumption by any social group.
20 Before starting a drug therapy, a number of criteria should be taken into account (age of the mother, comorbidities, and even other non-drug alternatives) to avoid undesirable events,
17 and through the aforementioned result it can be inferred that prescriptions are becoming less rigorous. Thus, the risks that most of these pregnancies take are avoidable. The measure is justifiable, in some case, when fetal exposure is necessary to guarantee mother’s health, otherwise non-drug therapies should be prioritized.
Despite it being common for pregnant women to use medications on their own for mild symptoms in pregnancy, self-medication has a tendency to decrease in the gestational period compared with the previous period.
21 This explains the fact that the self-medication reported in this study was 2%. This situation may be due to a limitation of the study, where the pregnant women may be apprehensive to report poor health practices. Another Brazilian study also presented a similar frequency.
11 Nonetheless, the existence of self-medication practices shows weaknesses in the provision of education services regarding the safety of medications in pregnancy.
With regard to the classifications of those used during pregnancy, antianemics had the highest frequency of use. This reinforces the idea that the WHO guidelines on folic acid and ferrous sulfate supplementation in pregnancy contribute to the use of those medications by that public.
22 The literature also indicates the same prevalence for antianemics.
11,16 Folic acid has been indicated as an important drug with protective action against neural tube defects, with preventive effects against a low birth weight and damage to fetal growth; the clinical relevance of ferrous sulfate lies in it being a prophylactic agent against gestational anemia, guaranteeing protection against pathological consequences of iron deficiency.
23Besides certain medications containing some vitamins indicated in pregnancy, ascorbic acid and ondansetron were among the most used for the gastrointestinal tract. Although the use of ascorbic acid is related to the prevention of some complications in pregnancy, there is little confidence about whether mothers are exempt from the potential risks and so the guidance is to use with caution and seek medical advice.
24 Regarding ondansetron, in 2019, Anvisa published a warning for pregnant women and health professionals about new discoveries of the risks of the drug. Generally used for treating nausea and vomiting, it has been associated with high risks of defects in fetal formation, particularly the emergence of orofacial clefts.
25Drugs that act in the central nervous system (CNS) were the third most used class by the pregnant women. Paracetamol, the most used analgesic in pregnancy, presents greater safety compared with dipyrone, given that no association has been found with flaws in fetal development or fetotoxicity.
18 On the other hand, questions have been raised about the safety of using acetylsalicylic acid (ASA). It has the second highest prevalence among the CNS drugs and there is a suggested association with early kidney problems and cryptorchidism (incorrect positioning of the male sexual gonads).
26 In addition, one case-control study showed that when used during pregnancy this drug was associated with the occurrence of hearing loss in the child.
27Most medications used before pregnancy were classified by the FDA as risk C in the first months of pregnancy and contraindicated by Anvisa. This situation is explained because most women were not aware of their pregnancy and so used contraindicated medications. Prominent among the medications used (besides dipyrone in isolation) was orphenadrine associated with caffeine and dipyrone. In addition, medications with an X risk also presented high prevalence in that period, which may be explained by the concerning use of contraceptives previously discussed. Likewise, they include those that are fundamental for some chronic diseases, thus a re-evaluation of their use after the pregnancy diagnosis is expected.
11In the pregnancy period, most of the medications mentioned were classified as risk A and indicated by Anvisa. Antianemics belong to that category and so the prevalence reinforces the point discussed about the importance of antianemic use in pregnancy. Thus, it is perceived that professionals are concerned about maintaining conditions for healthy development. However, after these, the most frequent ones were considered as use with caution and risk C, showing that there are prescriptions with questionable safety for fetal formation. Based on that, the situation suggests that the medications may have been inappropriately chosen without the application of a risk-benefit assessment.
16During pregnancy, the risk C medications remained frequent. Scopolamine in isolation and/or associated with paracetamol was one of the prominent ones and represented 4.1% of the medications used and contraindicated. The studies conducted to evaluate scopolamine exposure are rare, but there are suggestions that it may cause some congenital malformations.
28In the present study, having a family income below or equal to one minimum wage was associated with medication use before pregnancy. Other studies
11,20 presented the opposite result, in which those with a higher income were associated with use. However, the availability of free medications through the SUS means there is little influence of financial inequality on access.
29 In addition, it is suggested that pregnant women that have a low income may be more exposed to unfavorable conditions that affect their state of health and thus need greater pharmacological intervention.
With regard to maternal factors, it was possible to observe that the mothers that had a health problem and complaints during pregnancy were associated with medication use in that period. In addition, they presented greater consumption, consistently with the data found in the literature.
10,20 This situation shows that pregnant women have a medicalized view of their mild grievances and symptoms, with medication being the path for an immediate cure.
4 Besides that culture among pregnant women, health professionals also contribute to that situation due to their perceptions of pharmacological interventions being the product of their knowledge. Similarly, the higher number of consultations, even though only showing a significant association in the crude analysis, represents greater contact with the prescribing professional, enabling access to the medications through the pre-natal care itself.
30The use of a form to collect information on medication use based solely on participant self-reporting served to include data on self-medication. However, this type of collection cannot determine the situation of rational use of medications or therapeutic adhesion and may be influenced by possible memory bias. In addition, interviews that were not carried out in a reserved environment could lead to suspicions that the participants may omit information. Likewise, failing to set a gestational period as a criterion for inclusion in the research may have generated a confounding factor for some variable.
Therefore, the study was shown to be capable of characterizing medication use by pregnant women in one municipality in Brazilian Northeast. There is a tendency for flaws in the family planning carried out by the FHS units, which caused a high rate of exposure to the risks of using some toxic substance in periods close to conception. Equally, throughout the whole gestational period, that same situation highlights oversights in the pre-natal care and a low appropriation of knowledge about medication use in pregnancy.
Therefore, the responsibilities for minimizing risks are attributed both to the professionals and to pregnant women and community. The health team, including the pharmacist, should prioritize the use of soft technologies (listening, speaking, bonding, hospitality) to treat complications in pregnancy, seeking the best maternal and infant health conditions. For those that really need pharmacological treatment, there should always be a thorough re-evaluation of the medications to be prescribed, taking into account the risk-benefit. Moreover, it is up to the pharmacist to carry out the pharmacotherapeutic monitoring of these pregnant women in order to promote their wellbeing. Pregnant women should also be included in the care process for the formation and strengthening of ties that enable the promotion of and education in health.
References1. Ministério da Saúde (BR). Portaria n. 1.459, de 24 de junho de 2011. Institui no âmbito do Sistema Único de Saúde-SUS-a Rede Cegonha. Brasília (DF): DOU de 24 de junho 2011. [access on 2021 Jun 6]. Available at:
https://bvsms.saude.gov.br/bvs/saudelegis/gm/2011/prt1459_24_06_2011.html2. Venancio SI, Rosa TEC, Sanches MTC, Shigen EY, Souza JMP. Efetividade da Estratégia Saúde da Família sobre indicadores de saúde da criança no Estado de São Paulo. Rev Bras Saúde Matern Infant. 2016; 16 (3): 283-93.
3. Vieira LS, Abreu JA, Paulo KKS, Menezes LS, Martins LB, Comper MLC. Gestação, parto e puerpério na perspectiva das gestantes de uma unidade básica de saúde. Rev Revise. 2020; 4 (0): 116-31.
4. Geib LTC, Filho EFV, Geib D, Mesquita DI, Nunes ML. Prevalência e determinantes maternos do consumo de medicamentos na gestação por classe de risco em mães de nascidos vivos. Cad Saúde Pública. 2007; 23 (10): 2351-62
5. Burkey BW, Holmes AP. Evaluating medications use in pregnancy and lactation: what every pharmacist should know. J Pediatr Pharmacol Ther. 2013; 18 (3): 247-58.
6. Vargesson N. Thalidomide-induced teratogenesis: history and mechanisms. Birth Defects Res C Embryo Today. 2015 Jun; 105 (2): 140-56.
7. Sachdeva P, Patel BG, Patel BK. Drug use in pregnancy: a point to ponder! Indian J Pharm Sci. 2009; 71 (1): 1-7
8. Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária (ANVISA). Resolução nº 60, de 17 de dezembro de 2010. Estabelece frases de alerta para princípios ativos e excipientes em bulas e rotulagem de medicamentos. Brasília (DF): DOU de 17 de dezembro de 2010. [access on 2021 Jun 6]. Available at:
https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2010/res0060_17_12_2010.html#:~:text=Estabelece%20frases%20de%20alerta%20para,bulas%2 0e%20rotulagem%20de%20medicamentos.&text=3%C2%BA%20do%20art.,Anexo%20I%20da%20Portaria%20n%C2%BA.9. Lupattelli A, Spigset O, Twigg MJ, Zagorodnikova K, Mardby AC, Moretti ME,
et al. Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open Qual. 2014; 4 (2).
10. Costa DB, Coelho HLL, Santos DB. Utilização de medicamentos antes e durante a gestação: prevalência e fatores associados. Cad Saúde Pública. 2017; 33 (2): 1-14.
11. Andrade AM, Ramalho AA, Koifman RJ, Dotto LMG, Cunha MA, Opitz SP. Fatores associados ao uso de medicamentos na gestação em primigestas no Município de Rio Branco, Acre, Brasil. Cad Saúde Pública. 2014; 30 (5): 1042-56.
12. Ministério da Saúde (BR). E-Gestor: atenção básica. Informação e gestão da atenção básica. [Internet] [access on 2020 Jul 28]. Available at:
https://egestorab.saude.gov.br/index.xhtml13. Rede Interagencial de Informação para a Saúde (RIPSA). Indicadores de mortalidade infantil. [Internet] [access on 2020 Jul 28]. Available at:
http://www3.saude.ba.gov.br/cgi/tabcgi.exe?tabnet/ripsa/c01/c01a.def14. WHO International Working Group for Drug Statistics Methodology; WHO Collaborating Centre for Drug Statistics Methodology; WHO Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Services. Introduction to drug utilization research. Geneva: WHO; 2003. [access on 2020 Jul 28]. Available at:
https://apps.who.int/iris/handle/10665/4262715. Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária. Bulário eletrônico [Internet]. Brasília (DF): Ministério da Saúde; 2013 [accessed on 2020 Aug 28]. Available at:
http://www.anvisa.gov.br/datavisa/fila_bula/index.asp16. Maia LT, Trevisol FS, Galato D. Uso de medicamentos no primeiro trimestre de gravidez: avaliação da segurança dos medicamentos e uso de ácido fólico e sulfato ferroso. Rev Bras Ginecol Obstet. 2014; 36 (12): 541-7.
17. Tacon FSA, Amaral WN, Tacon KCB. Medicamentos e gravidez: influencia na morfologia fetal. Rev Educ Saúde. 2017; 5 (2): 105-11.
18. Couto AC, Ferreira JD, Oliveira MSP, Koifman S. Pregnancy, maternal exposure to analgesic medicines, and leukemia in Brazilian children below 2 years of age. Eur J Cancer Prev. 2015; 24 (3): 245-52.
19. Buur LE, Laurberg VR, Ernst A, Arendt LH, Andersen AMN, Olsen J,
et al. Oral contraceptive use and genital anomalies in sons: a Danish cohort study. Reprod Toxicol. 2019; 89: 67-73.
20. Costa KS, Barros MBA, Francisco PMSB, César CLG, Goldbaum M, Carandina L. Utilização de medicamentos e fatores associados: um estudo de base populacional no Município de Campinas, São Paulo, Brasil. Cad Saúde Pública. 2011; 27 (4): 649-58.
21. Silva LKP, Marques AEF. Utilização de medicamentos por gestantes: uma revisão sistemática da literatura. Rev Aten Saúde. 2019; 17 (62): 90-7.
22. World Health Organization (WHO). Diretriz: Suplementação intermitente de ferro e ácido fólico em gestantes não anêmicas. Genebra: WHO; 2013. [access on 2020 Jul 28]. Available at:
http://apps.who.int/iris/bitstream/handle/10665/75335/9789248502019_por.pdf?sequence=923.Pereira RA, Teles JN, Costa CML. A importância do ácido fólico e sulfato ferroso na gestação. Rev Extensão. 2019; 3 (1): 75-82.
24. Rumbold A, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database Syst Rev. 2005 Apr; 2: CD004072.
25. Agência Nacional de Vigilância Sanitária (ANVISA). Uso de ondasetrona é investigado. [
Internet] 2019. [access on 2020 Jul 30]. Available at:
http://antigo.anvisa.gov.br/resultado-de-busca?p_p_id=101&p_p_lifecycle=0&p_p_state=maximized&p_p_mode=view&p_p_col_id=column-1&p_p_col_count=1&_101_struts_action=%2Fasset_publisher%2Fview_content&_101_assetEntryId=5650124&_101_type=content&_101_ groupId=219201&_101_urlTitle=uso-de-ondansetrona-e-investigado&inheritRedirect=true26. Bloor M, Paech M. Nonsteroidal anti-inflammatory drugs during pregnancy and the initiation of lactation. Anest Analg. 2013; 116 (5): 1063-75.
27. Foch C, Araujo M, Weckel A, Michel CD, Montastruc JL, Benevent J,
et al. In utero drug exposure and hearing impairment in 2-year-old children a case-control study using the EFEMERIS database. J Pediatr Otorhinolaryngol. 2018 Oct; 113: 192-7.
28. Lee NM, Sumona S. Nausea and vomiting of pregnancy. Gastroenterol Clin. 2011; 40 (2): 309-34.
29. Ferreira RA, Barreto SM, Giatti L. Hipertensão arterial referida e utilização de medicamentos de uso contínuo no Brasil: um estudo de base populacional. Cad Saúde Pública. 2014; 30 (4): 815-26.
30. Fonseca MRCC, Fonseca E, Mendes GB. Prevalência do uso de medicamentos na gravidez: uma abordagem farmacoepidemiológica. Rev Saúde Pública. 2002; 36 (2): 205-12.
Received on March 16, 2021
Final version presented on April 27, 2022
Approved on September 27, 2022
Authors’ contribution: Campos HMN, Mattos MP, and Gomes DR participated in the data analysis and critical review and approved the final version of the article.
The authors declare that there are no conflicts of interest.
 ; Mússio Pirajá Mattos2
; Mússio Pirajá Mattos2 ; Daiene Rosa Gomes3
; Daiene Rosa Gomes3