ABSTRACT
OBJECTIVES: to evaluate the association between alcohol and tobacco consumption during pregnancy with maternal and child health conditions.
METHODS: cross-sectional study with a probabilistic sample of pregnant women living in Santa Catarina who conducted prenatal care and childbirth in the public national health service in 2019. A face-to-face survey questionnaire was applied to 3,580 pregnant women including maternal health issues during pregnancy and perinatal health of the newborn. Crude logistic regression analyzes were performed and adjusted for socio-demographic and maternal health conditions.
RESULTS: the prevalence of alcohol and tobacco consumption during pregnancy was 7.2% and 9.3%, respectively. Alcohol consumption during pregnancy increased the chance of maternal anemia by 45% (CI95%=1.09-1.91), increased the chance of gestational diabetes by 73% (CI95%=1.14-2.63) and reduced the chance of hypertension (OR=0.59; CI95%=0.37-0.94). Tobacco consumption doubled the chance of low birth weight (OR=2.16; CI95%=1.33-3.51).
CONCLUSION: the consumption of alcoholic beverages during pregnancy increased the chance of maternal health complications, such as anemia and gestational diabetes, while tobacco increased the chance of low birth weight.
Keywords:
Alcohol drinking, Smoking, Pregnancy, Maternal and child health
RESUMO
OBJETIVOS: avaliar a associação entre o consumo de álcool e de tabaco durante a gravidez com condições de saúde maternas e da criança.
MÉTODOS: estudo transversal com amostra probabilística de gestantes residentes em Santa Catarina que realizaram o pré-natal e o parto na rede pública do estado em 2019. Foi aplicado questionário face-a-face com 3.580 gestantes incluindo questões de saúde maternas durante a gestação e saúde perinatal do recém-nascido. Foram realizadas análises de regressão logística brutas e ajustadas para condições sócio-demográficas e de saúde maternas.
RESULTADOS: as prevalências de consumo de bebidas alcoólicas e de tabaco durante a gestação foram de 7,2% e 9,3%, respectivamente. O consumo de álcool durante a gestação aumentou em 45% a chance de anemia materna (IC95%=1,09-1,91) e em 73% a de diabetes gestacional (IC95%=1,14-2,63) e reduziu a chance de hipertensão (OR=0,59; IC95%=0,37-0,94). O consumo de tabaco dobrou a chance de baixo peso gestacional ao nascer (OR=2,16; IC95%=1,33-3,51).
CONCLUSÃO: o consumo de bebidas alcoólicas durante a gestação aumentou a chance de intercorrências de saúde maternas, como anemia e diabetes gestacional, enquanto o tabaco aumentou a chance de baixo peso ao nascer.
Palavras-chave:
Consumo de bebidas alcoólicas, Fumar, Gravidez, Saúde materno-infantil
IntroductionAlcohol and tobacco consumption in societygenerates great impacts to the public health and represents a major aggravating factor to women in gestational period, and may negatively affect fetal and embryonic development, as well as outcomes in infant health. Some harms caused by the exposition to these teratogens may remain for the entire life of the individual.
1-3Alcohol ingestion and the use of cigarettes in pregnancy vary broadly among different countries, although, for both of them, the estimated prevalence in Brazil is higher than the global average. It is estimated that in the country, in 2012, 15.2% of pregnant women consumed alcoholic beverages during pregnancy and 9.6% reported the habit of smoking during this period.
4,5 The prevalence of tobacco exposition may be even higher considering passive smokers.
6The prenatal exposure to alcohol may lead to countless adverse effects to the fetal development, depending on the gestational trimester and the consumed dose. Alcohol increases the risk of abortion, of preterm births and restrictions in fetal growth.
3,7 Besides, it may lead to Fetal Alcohol Syndrome (FAS), which normally is not diagnosed in the first years of life, but causes, within other conditions, physical and behavioral alterations and intellectual disability.
8As well as alcohol, tobacco may also lead to several negative consequences to fetal and pregnant women health, such as postnatal growth retardation, placental abnormalities, preterm births, higher rates of spontaneous abortions, cognitive disorders, respiratory diseases and pulmonary function reduction.
9,10In Brazil, the literature that investigated the subject was mostly published with non-probabilistic sample, restricted to few municipalities and, in most cases, with a convenience selection from only one hospital.
11-13 In addition, systematic reviews about the influence of alcohol consumption in pregnancy point to the importance of the conduction of studies with adjustment for socioeconomic variables, being this a limitation in different published studies.
14 Concerning tobacco in pregnancy, the review carried out by Salmasi
et al.
6 points to the need for further studies, also highlighting the importance of including confounding variables in the analyses.
This study aimed to analyze the association between alcohol and tobacco consumption in pregnancy with different mother and child outcomes, in a sample with puerperal women assisted in the Unified Health System (SUS - Portuguese acronym).
MethodsCross-sectional study with a probabilistic sample of puerperal women that performed prenatal consultations and delivery in the SUS in the state of Santa Catarina in 2019. Data collection was performed in state public hospitals with puerperal women that agreed to answer the questionnaire of research. The inclusion criteria were: having resided in Santa Catarina during the entire pregnancy; having performed all prenatal consultations in the public health network (both habitual risk and high risk pregnancies); having performed the delivery in maternity hospitals located in Santa Catarina; having had live birth, stillbirth or child dead up to 48 hours postpartum, and who were born weighing more than 500g and with at least 22 weeks of pregnancy. The definition of hospitals used for data collection followed as a criterion having performed more than 500 deliveries by the SUS in the year 2016, which corresponded to 86.2% of all births in the state funded by the SUS.
The total sample of the study was estimated in 3,665 puerperal women to be interviewed, being considered for the calculation a confidence interval of 95%, margin of error of 1.6%, population size of 50 thousand people and estimated prevalence of the phenomenon of 50%, being added 5% to the total of the sample to include losses and refusals.
For data collection, closed face-to-face questionnaires were used in the hospitals up to 48 hours postpartum by means of tablets and with registry in REDCap platform and some data were collected directly from medical records and pregnant women's booklet. The field logistics was tested in a study pilot with 5% of the sample. In total, 35 interviewers, all of them with higher education or attending university and with experience in the health area, were qualified and participated in data collection. The quality control was performed with random sampling of 10% of the interviewees by phone. All variables of quality control demonstrated good or almost perfect concordance, and the analyzed variables used in the present study showed Cohen's Kappa coefficient above 0.680. Broader methodological details can be obtained in the article by Boing
et al.
15Self-reported dependent variables included maternal health conditions: presence or absence of anemia, gestational diabetes, gestational hypertension, placenta previa, placental abruption and bleeding in the last 3 months. Were analyzed as perinatal and delivery conditions: low birth weight according to criteria of the World Health Organization (<2.5 kg),
16 prematurity (gestational age <37 weeks) - both obtained from the health booklet -, and the type of delivery, self-reported by the puerperal woman. Were considered as independent variables of main interest, alcohol and tobacco consumption during pregnancy, categorized in yes or no. For alcohol consumption, the participant was asked whether she got used to drinking alcoholic beverages during pregnancy, and concerning tobacco consumption, the participant was asked whether she had smoked or if smoked during pregnancy. Participants that consumed tobacco at any moment of pregnancy were analyzed.
The adjustment variables were age group (13-19, 20-35 and 36-46 years), marital status (married, stable union, single, divorced or widow), skin color/self-reported race (white, black and brown), years of schooling (≥13, 10-12, ≤9),
per capita income in tertiles. As adjustment variables for maternal health, were considered parity (with or without other children), physical activity practice before pregnancy (yes; no), and Body Mass Index before pregnancy (categorized low/normal; overweight; obesity). Initially, sample distribution, prevalence, and its respective confidence intervals of 95% (CI95%) of outcomes according to the presence or not of maternal health conditions and prenatal characteristics were calculated. In sequence, odds ratio were calculated by means of logistic regression in crude and adjusted models, and the measures reported with their respective CI95%, and considered statistically significant when
p<0.05. All analyses were performed with Stata 15.1 software.
The research was approved by the Research and Ethics Committee (CEP - Portuguese acronym) of the Federal University of Santa Catarina under opinion number 1.599.464.
ResultsA total of 3,580 puerperal women answered to the questionnaire, representing 97.7% of women that met the inclusion criteria. In Table 1, characteristics of these participants are described. It was verified that 2 of 3 women (66%) were between 20 and 34 years old, 78.6% were married or living with their partner, around half had complete/incomplete high school and 63.4% were white. The reported prevalence of alcohol consumption during pregnancy was 7.2% (CI95% 6.4 - 8.1) and of tobacco consumption, 9.3% (CI95%=8.4-10.3) (Table1).
Among maternal and perinatal health characteristics analyzed, 5.2% of the sample had low birth weight, and 7.7% were preterm. The type of delivery was 57.1% vaginal and 42.9% cesarean. Gestational anemia was reported in 38.9% of interviewees, gestational diabetes in 9.4% and hypertension in 15.8% (Figure 1).
In Table 2 it is possible to verify that among women who consumed alcohol in pregnancy, the prevalence of gestational anemia was 46.8%, of gestational diabetes, 13.2% and hypertension, 10.6%. Among those who reported smoking, we highlight that 1 of 10 babies were born with low weight (Table 2).
When analyzing alcohol consumptions in all outcomes, the crude and adjusted analyses had the same associations. In the adjusted analyses, it was verified 45% more chances of gestational diabetes (CI95%=1.09-1.91) and 73% more chances of gestational diabetes (CI95%- 1.14-2.63) among women who reported alcohol consumption compared to those who did not consume alcohol in pregnancy. It was also observed an association between alcohol consumption with the absence of gestational hypertension (OR=0.59; CI95%=0.37%-0.94) (Table 3).
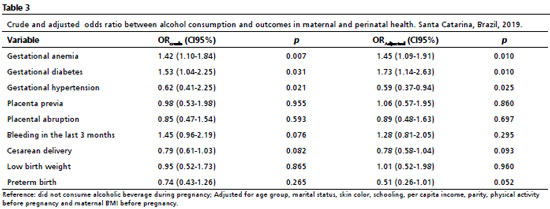
With regard to tobacco, in the crude analyses the variables "type of delivery" and "low birth weight" were associated with the outcome, with higher chance for low birth weight and vaginal delivery among pregnant women that smoked. In the adjusted analyses, the variable type of delivery lost statistical significance, but it was observed that tobacco consumption during pregnancy doubled the chance of low birth weight (OR=2.16, CI95%=1.33-3.51) (Table 4). Other analyzed outcomes did not have association with alcohol and tobacco consumption during pregnancy.
DiscussionThe present study observed an association between alcohol consumption during pregnancy with gestational diabetes, gestational anemia and gestational hypertension, whilst tobacco was associated with low birth weight of the newborn.
The national survey on alcohol and drugs demonstrated that there was an increase in alcohol consumption by the female public between 2010 and 2018, oscillating from 27% to 30%.
17 Between pregnant women, the national prevalence was 15.2% in 2012, being superior to what was observed in other American countries.
4 Concerning the prevalence of tobacco consumption among pregnant women, it varied from 4.1% and 16.5%, in a study carried out in three different Brazilian cities.
18 Pregnancy is a condition in which the cessation of these habits are encouraged, since the literature do not preconize any safe dose of alcohol or nicotine for the maternal and fetal health. However, the chances of women that consume alcohol to interrupt the ingestion are still low. In a maternity hospital in São Paulo, of 43.9% of women that consumed alcohol before pregnancy, 33.3% still consumed it in some moment during the pregnancy.
19In this study, alcohol consumption was associated with a higher chance of gestational anemia and diabetes. Pregnancy itself is an important risk factor for developing conditions such as diabetes, hypertension and anemia, being important factors to be controlled during prenatal consultations. The exposure to alcohol during pregnancy was previously associated with placental insufficiency, diminish of the size of placenta, blood flow and impaired transport of nutrients, endocrine alterations, increase in rates of stillbirths and placental abruption and vasoconstriction of the umbilical cord, which impairs the rate of alcohol elimination from the fetal compartment and low birth weight.
11 Besides, alcohol interferes in the metabolism of carbohydrates and glucose, leading to hypoglycemia as well as long-term problems in systemic arterial hypertension, weight gain and other comorbidities. There is also the aggravating factor of the interference of alcohol in nutrients absorption. Vitamin B12 or folic acid deficiency, in addition to the toxic effect of alcohol, may lead to macrocytic or megaloblastic anemia, for example.
21 The present study did not evaluate the weight gain or eating habits of pregnant women, however, alcohol consumption may predispose the occurrence of metabolic diseases, which, in turn, predispose the occurrence of diabetes.
The occurrence of gestational hypertension was lower with alcohol consumption. Even as anemia and diabetes, hypertension is associated with nutritional factors, or the disease itself established before the pregnancy. The causes of hypertension associated with pregnancy and preeclampsia are not fully understood, but there is strong evidence of genetic factors or gestational history.
22Some studies attempt to establish protector effects of determined standards of alcohol consumption in the cardiovascular system. However, studies of alcohol and hypertension are contradictory.
23 The damages of alcohol in fetal development, unequivocally, contraindicates the consumption even in minimal doses during pregnancy.
3 There is, thus, the need for discouraging the consumption of alcoholic beverages and tobacco among pregnant women, in order to prevent probable harms related to their consumption in individuals that were born from these pregnancies and for further awareness about the impact in public health.
Concerning perinatal and birth intercurrences, this study did not present a relationship between alcohol consumption and tobacco in the type of delivery or preterm birth. Regarding the variable "birth weight", alcohol consumption was not associated with this outcome, yet tobacco was associated with low birth weight. In a study carried out in the Netherlands between 2007 and 2010, birth weight, gestational age and weight for gestational age were not associated with alcohol consumption.
24 Studies had already demonstrated that the association of chemical dependency, such as alcohol to tobacco, or caffeine to tobacco, tends to increase the amount of consumption or its frequency. Alcohol and cigarette consumption exacerbated the adverse impact of such substances in parameters and in health status (birth weight, body length, Apgar score), as well as in the duration of pregnancy.
25The literature reports the risks of tobacco concerning spontaneous abortion, being the risk of abortion related to the amount of cigarettes.
26 Thus, the recommendation of discouraging the use of tobacco in pregnancy should be encouraged. Besides, negative outcomes of tobacco consumption include low birth weight, prenatal mortality and increase of the risk of sudden infant death syndrome.
27 In a prospective cohort study carried out in Dublin between 2010 and 2011, it was demonstrated the association between tobacco consumption and the restriction of intrauterine growth and the decrease of average birth weight.
28 The occurrence of low birth weight is not always associated with causes from maternal health, but healthy eating habits and weight gain in pregnancy are factors already associated with birth weight, for example.
29The limitations of the present cross-sectional study include recall bias, since the survey was performed after delivery, and the veracity and accuracy of participants in reporting alcohol and tobacco consumption. It is also considered as a limitation the fact that the amount of alcohol and tobacco consumed during pregnancy were not analyzed, and both women with daily alcohol and/or tobacco consumption and those with eventual consumption in pregnancy may participate in the same group. On the other hand, this is a probabilistic study and had data collection on gestational age and birth weight directly from the pregnant women's record, besides the representative puerperal women sample that performed prenatal consultations exclusively in the SUS of the entire state.
In conclusion, this study demonstrated that the consumption of alcoholic beverages during pregnancy is associated with gestational anemia and diabetes, whilst tobacco is associated with low birth weight. The planning of public policies of education and prenatal follow-up is important to mitigate the risks of morbidity in pregnant women, fetuses and newborns, as well as the costs of the health system with preventable causes of perinatal health, being the prenatal assistance an efficient strategy of providing explanations and support to pregnant women that need higher care with the abstinence. We highlight, in this sense, the importance of strategies that aim to reduce alcohol consumption in pregnancy. In the extent of SUS,
30 it is described the need for creating spaces of education in prenatal health, inside and outside the UBS (basic health units) and other health services, where they should figure as a place of discussion and orientations about life style.
References1. Queiroz Andrade E, Silva Sena CR, Collison A, Murphy VE, Gould GS, Bonevski B,
et al. Association between active tobacco use during pregnancy and infant respiratory health: a systematic review and meta-analysis. BMJ Open. 2020; 10 (9): e037819.
2. Baptista FH, Rocha KBB, Martinelli JL, Avó LRDSD, Ferreira RA, Germano CMR, Melo DG. Prevalência e fatores associados ao consumo de álcool durante a gravidez. Rev Bras Saúde Matern Infant. 2017; 17: 271-9.
3. Silva TP, Viana JSB, Silva AP, Silva BHFP, Silva GME, Moraes LDA,
et al. Fetal alcoholic syndrome and consequences on child neurodevelopment: a literature review. Res Soc Dev. 2022; 11 (5): e23511528091.
4. Lange S, Probst C, Heer N, Roerecke M, Rehm J, Monteiro MG,
et al. Actual and predicted prevalence of alcohol consumption during pregnancy in Latin America and the Caribbean: Systematic literature review and meta-analysis. Rev Panam Salud Publica. 2017; 8; 41: e89.
5. Domingues RMSM, Figueiredo VC, Leal MC. Prevalence of pre-gestational and gestational smoking and factors associated with smoking cessation during pregnancy, Brazil, 2011-2012. Plos One. 2019; 14 (5): e0217397.
6. Marufu TC, Ahankari A, Coleman T, Lewis S. Maternal smoking and the risk of still birth: systematic review and meta-analysis. BMC Public Health. 2015; 15: 239.
7. Wilson JG. Experimental studies on congenital malformations. J Chronic Dis. 1959; 10 (2): 111-30.
8. Murawski NJ, Moore EM, Thomas JD, Riley EP. Advances in diagnosis and treatment of fetal alcohol spectrum disorders: From animal models to human studies. Alcohol Res. 2015; 37 (1): 97-108.
9. Fried PA, O'connell CM, Watkinson B. 60− and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: Cognitive and language assessment. J Dev Behav Pediatr. 1992; 13 (6): 383-91.
10. Brion MJ, Victora C, Matijasevich A, Horta B, Anselmi L, Steer C,
et al. Maternal smoking and child psychological problems: Disentangling causal and noncausal effects. Pediatrics. 2010 Jul; 126 (1): 57-65.
11. Sbrana M, Grandi C, Brazan M, Junquera N, Nascimento MS, Barbieri MA,
et al. Consumo de álcool durante a gravidez e resultados perinatais: Um estudo de coorte. Sao Paulo Med J. 2016 Mar; 134 (2): 146-52.
12. Maria FN, Jornada LK, Sakae TM, Cassol-Jr OJ, Sakae DY, Quevedo JL. Uso de álcool e tabaco por gestantes em maternidade do sul de Santa Catarina. Arq Catarin Med. 2015; 44 (1): 41-61.
13. Hackbarth BB, Ferreira JA, Carstens HP, Amaral AR, Silva MR, Silva JC,
et al. Suscetibilidade à prematuridade: Investigação de fatores comportamentais, genéticos, médicos e sociodemográficos. Rev Bras Ginecol Obstet. 2015 Ago; 37 (8): 353-8.
14. Mamluk L, Edwards HB, Savović J, Leach V, Jones T, Moore THM,
et al. Low alcohol consumption and pregnancy and childhood outcomes: Time to change guidelines indicating apparently "safe" levels of alcohol during pregnancy? A systematic review and meta-analyses. BMJ Open. 2017; 3; 7 (7): e015410.
15. Boing AF, Lacerda JT, Boing AC, Calvo MCM, Saraiva S, Tomasi YT,
et al. Métodos e aspectos operacionais de um estudo epidemiológico e de avaliação da Rede Cegonha. Rev Bras Epidemiol. 2021; 24: e210010.
16. World Health Organizantion (WHO). Physical status: The use and interpretation of anthropometry: report of a WHO expert committee. Geneva: WHO; 1995. [acesso em 2022 mar 12]. Disponível em:
https://apps.who.int/iris/handle/10665/3700317. Andrade AG. Álcool e a Saúde dos Brasileiros: Panorama. 1
st ed. São Paulo: Centro de Informações sobre Saúde e Álcool (CISA); 2020. [access in 2022 mar 12]. Available from:
https://cisa.org.br/images/upload/Panorama_Alcool_Saude_CISA2020.pdf18. Loret de Mola C, Cardoso VC, Batista R, Gonçalves H, Saraiva MCP, Menezes AMB. Maternal pregnancy smoking in three Brazilian cities: trends and differences according to education, income, and age. Int J Public Health. 2020; 65 (2): 207-15.
19. Mesquita MDA, Segre CAM. Frequência dos efeitos do álcool no feto e padrão de consumo de bebidas alcoólicas pelas gestantes de maternidade pública da cidade de São Paulo. Rev Bras Crescimento Desenvolvimento Hum. 2009; 19 (1): 63-77.
20. Sociedade Brasileira de Diabetes. Diretrizes da sociedade brasileira de diabetes 2019-2020. São Paulo: Clannad; 2020. [access in 2022 mar 12]. Available from:
https://portaldeboaspraticas.iff.fiocruz.br/biblioteca/diretrizes-da-sociedade-brasileira-de-diabetes-2019-2020/21. Lieber CS. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res Health. 2003; 27 (3): 220-31.
22. Amro F, Sibai B. Management of hypertension in pregnancy. Semin Fetal Neonatal Med. 2020. 25 (5): 101147.
23. Sesso HD, Cook NR, Buring JE, Manson JAE, Gaziano JM. Alcohol consumption and the risk of hypertension in women and men. Hypertension. 2008 Apr; 51 (4): 1080-87.
24. Lanting CI, Van Dommelen P, Van Der Pal-De Bruin KM, Bennebroek Gravenhorst J, Van Wouwe JP. Prevalence and pattern of alcohol consumption during pregnancy in the Netherlands. BMC Public Health. 2015; 15 (1): 723.
25. Hamułka J, Zielińska MA, Chądzyńska K. The combined effects of alcohol and tobacco use during pregnancy on birth outcomes. Rocz Panstw Zakl Hig. 2018; 69 (1): 45-54.
26. Pineles BL, Park E, Samet JM. Systematic Review and Meta-Analysis of Miscarriage and Maternal Exposure to Tobacco Smoke During Pregnancy. Am J Epidemiol. 2014; 179 (7): 807-23.
27. Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant Morbidity and Mortality Attributable to Prenatal Smoking in the U.S. Am J Prev Med. 2010; 39 (1): 45-52.
28. Murphy DJ, Dunney C, Mullally A, Adnan N, Deane R. Population-based study of smoking behaviour throughout pregnancy and adverse perinatal outcomes. Int J Environ Res Public Health. 2013; 10 (9): 3855-67.
29. Santana JM, Assis AMO, Alves WPO, Santos DB. Association between gestational weight gain and birt6h weight: Nisami cohort. Rev Bras Saúde Matern Infant. 2020; 20 (2): 411-20.
30. Ministério da Saúde (BR). Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Atenção ao pré-natal de baixo risco. Brasília (DF): Ministério da Saúde; 2012. [access in 2022 Jan 15]. Available from:
https://bvsms.saude.gov.br/bvs/publicacoes/cadernos_atencao_basica_32_prenatal.pdfReceived on March 26, 2021
Final version presented on September 1, 2022
Approved on October 8, 2022
Associated Editor: Aurélio Costa
Author's contribution: Pavesi EP and Wagner KJP: analysis and data interpretation, elaboration and critical review of the manuscript. Amorim MVS: elaboration and critical review of the manuscript. Boing AF: project conceptualization, elaboration and critical review of the manuscript.
The authors approved the final version of the article and declare no conflict of interest.
 ; Marina Veiga da Silva Amorim2
; Marina Veiga da Silva Amorim2 ; Antonio Fernando Boing3
; Antonio Fernando Boing3 ; Katia Jakovljevic Pudla Wagner4
; Katia Jakovljevic Pudla Wagner4